Cancer Res Treat.
2019 Jul;51(3):1052-1063. 10.4143/crt.2018.411.
Investigating Trk Protein Expression between Oropharyngeal and Non-oropharyngeal Squamous Cell Carcinoma: Clinical Implications and Possible Roles of Human Papillomavirus Infection
- Affiliations
-
- 1Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. soyoon@yuhs.ac
- 2Yonsei University College of Medicine, Seoul, Korea.
- 3Division of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2454297
- DOI: http://doi.org/10.4143/crt.2018.411
Abstract
- PURPOSE
The relationship between head and neck squamous cell carcinoma (HNSCC) and subtypes of tropomyosin-related kinase (Trk) has not been studied in-depth. In this study, we evaluated the expression patterns of TrkA, TrkB, and panTrk and their clinicopathological significance as well as association with p16 expression and human papilloma virus (HPV) status.
MATERIALS AND METHODS
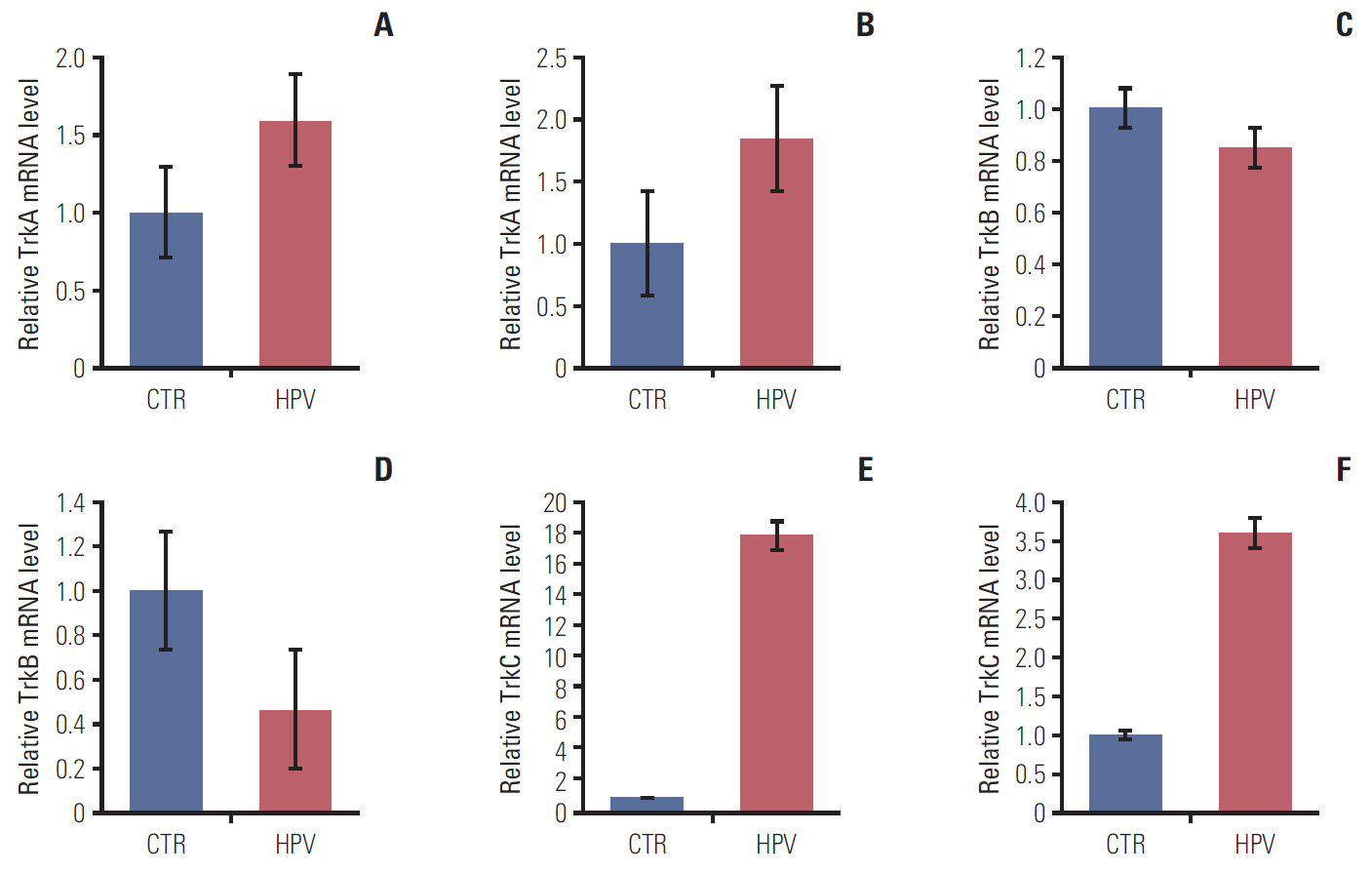
Total of 396 radically resected oropharyngeal (n=121) and non-oropharyngeal (n=275) HNSCCs were included. Immunohistochemistry for TrkA, TrkB, and panTrk was performed. In addition, p16 immunohistochemistry was performed to assess the HPV status. Using HPV-negative HNSCC cell lines, FaDu and CAL27, HPV type 16 E6/E7 gene was transfected, and then changes of TrkA and TrkB expression were analyzed.
RESULTS
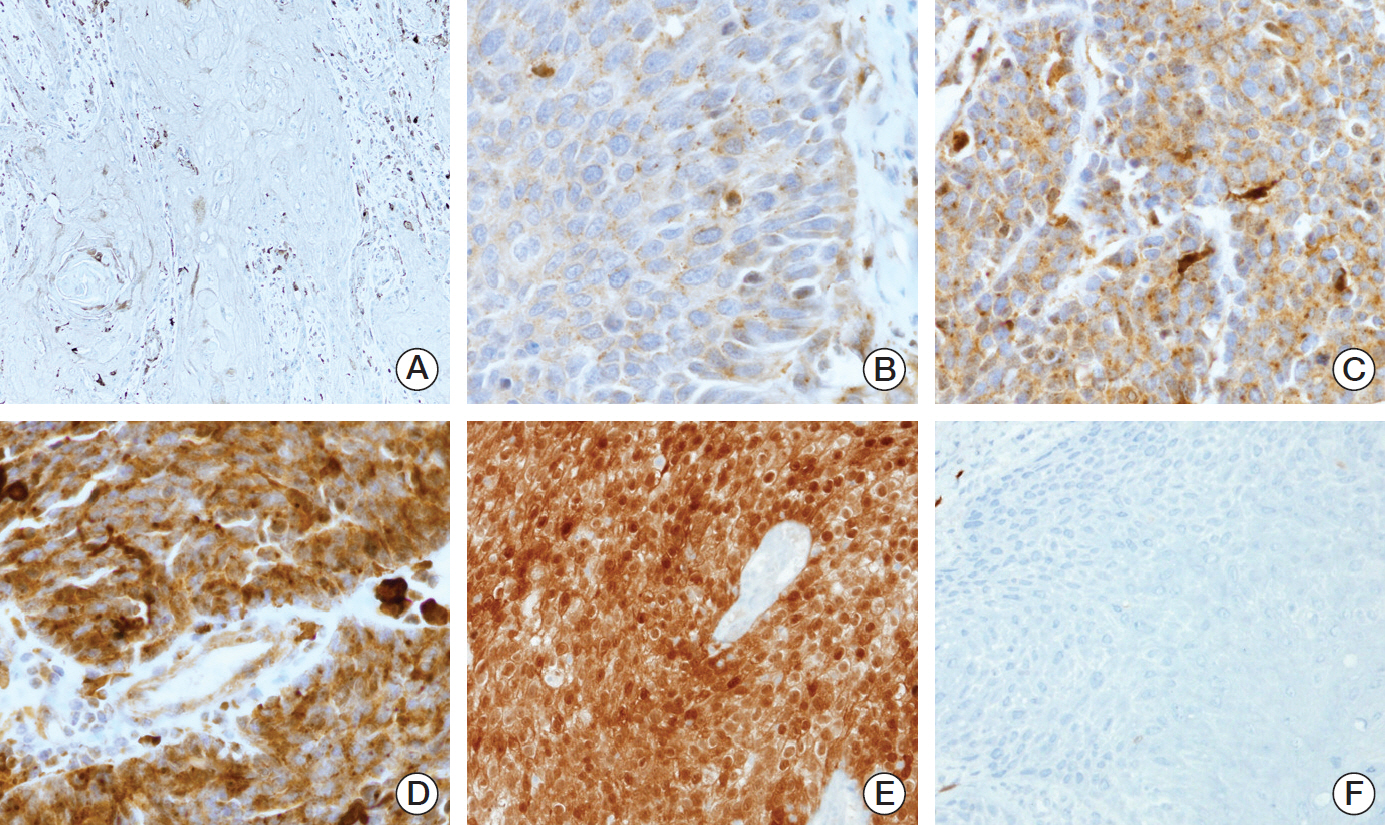
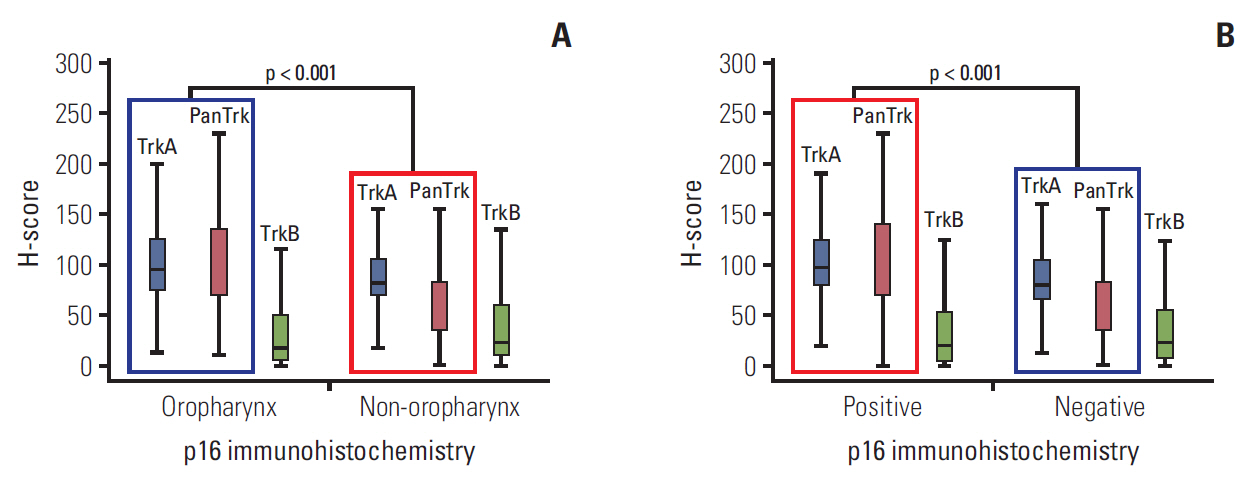
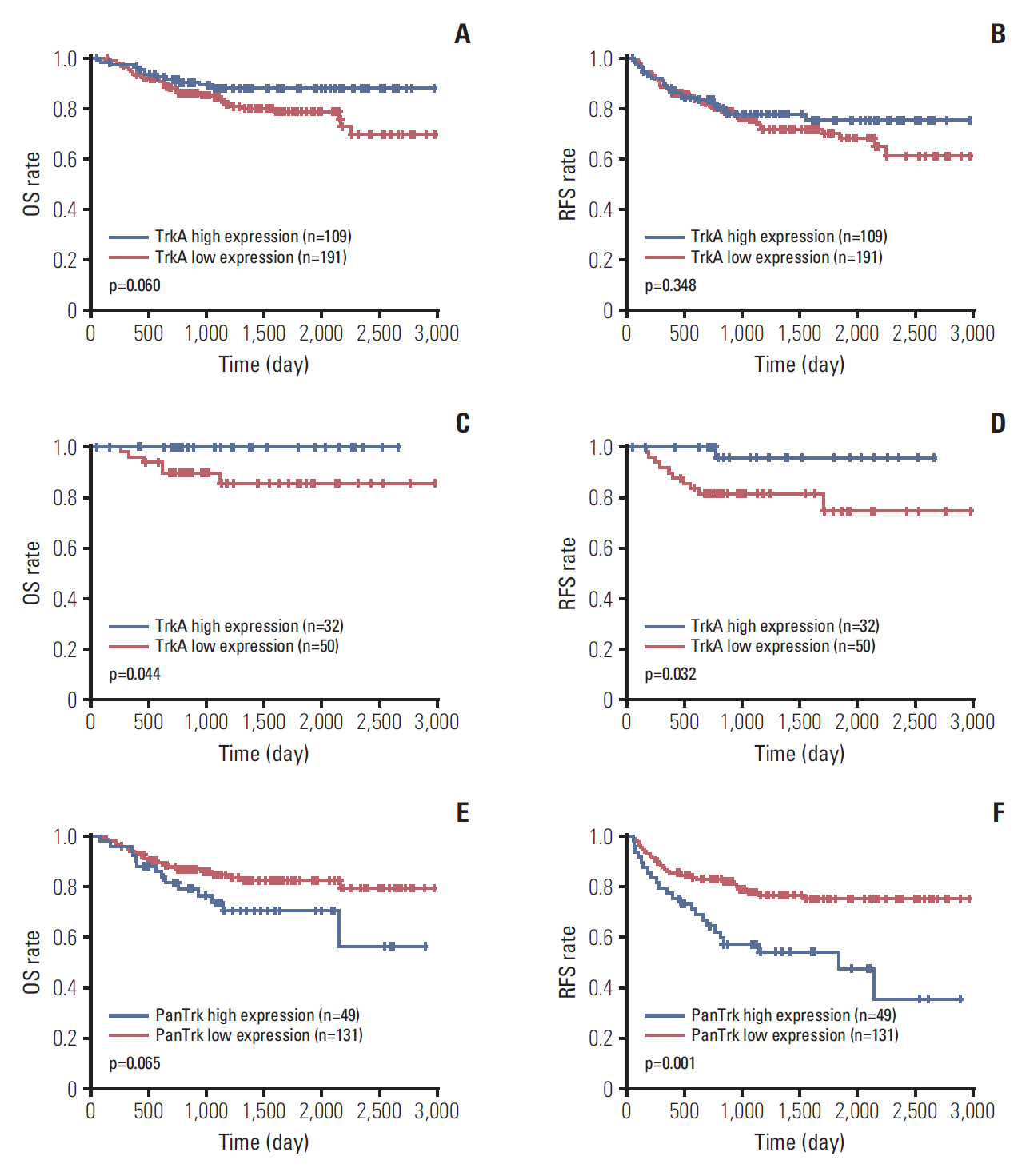
In the clinical samples of HNSCC, high expression of TrkA and panTrk were more associated with oropharyngeal and p16 positive squamous cell carcinoma (SCC). In patients with completely resected (R0-resected) oropharyngeal SCC, high TrkA expression was related to superior overall survival and recurrence-free survival (RFS). In patients with R0-resected oral cavity SCC, high panTrk was related to poor RFS. In HPV type E6/E7 gene-transfected FaDu and CAL27 cell lines, increase of TrkA expression was observed.
CONCLUSION
It seems that expression pattern of panTrk and TrkA differed according to anatomical sites of HNSCC and was closely related to p16 expression and patient prognosis. Trk expression should be considered in the context of anatomical site, p16 expression or HPV status and Trk subtypes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Ferroportin and FBXL5 as Prognostic Markers in Advanced Stage Clear Cell Renal Cell Carcinoma
Cheol Keun Park, Jayoon Heo, Won Sik Ham, Young-Deuk Choi, Sang Joon Shin, Nam Hoon Cho
Cancer Res Treat. 2021;53(4):1174-1183. doi: 10.4143/crt.2021.031.
Reference
-
References
1. Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013; 31:4550–9.
Article2. Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011; 129:2337–48.
Article3. Hamilton D, Khan MK, O'Hara J, Paleri V. The changing landscape of oropharyngeal cancer management. J Laryngol Otol. 2017; 131:3–7.
Article4. Westra WH, Lewis JS Jr. Update from the 4th Edition of the World Health Organization classification of head and neck tumours: oropharynx. Head Neck Pathol. 2017; 11:41–7.
Article5. Westra WH, Boy S, El-Mofty SK, Gillison M, Schwartz MR, Syrjanen S, et al. Squamous cell carcinoma, HPV-positive. In : El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. 4th ed. Lyon: IARC Press;2017. p. 136–8.6. Lewis JS Jr, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018; 142:559–97.
Article7. Fakhry C, Lacchetti C, Rooper LM, Jordan RC, Rischin D, Sturgis EM, et al. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement of the College of American Pathologists guideline. J Clin Oncol. 2018; 36:3152–61.
Article8. Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009; 15:5962–7.9. Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010; 29:2047–59.
Article10. Zhu L, Werner JA, Mandic R. Implications of tropomyosin-related kinase B (TrkB) in head and neck cancer. Anticancer Res. 2007; 27:3121–6.11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.12. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classfication of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press;2005.13. Cho YA, Kim EK, Heo SJ, Cho BC, Kim HR, Chung JM, et al. Alteration status and prognostic value of MET in head and neck squamous cell carcinoma. J Cancer. 2016; 7:2197–206.
Article14. Yun S, Koh JM, Lee KS, Seo AN, Nam KH, Choe G. Expression of c-MET in invasive meningioma. J Pathol Transl Med. 2015; 49:44–51.
Article15. Shin JH, Kim CJ, Jeon EJ, Sung CO, Shin HJ, Choi J, et al. Overexpression of C-reactive protein as a poor prognostic marker of resectable hepatocellular carcinomas. J Pathol Transl Med. 2015; 49:105–11.
Article16. Park E, Park SY, Kim H, Sun PL, Jin Y, Cho SK, et al. Membranous insulin-like growth factor-1 receptor (IGF1R) expression is predictive of poor prognosis in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma. J Pathol Transl Med. 2015; 49:382–8.17. Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012; 36:1874–82.
Article18. Mirghani H, Casiraghi O, Amen F, He M, Ma XJ, Saulnier P, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol. 2015; 28:1518–27.
Article19. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010; 363:24–35.
Article20. Lewis JS Jr, Chernock RD, Ma XJ, Flanagan JJ, Luo Y, Gao G, et al. Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol. 2012; 25:1212–20.
Article21. Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016; 108:djv403.22. Lewis JS Jr. p16 immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012; 6 Suppl 1:S75–82.
Article23. Choi Y, Won YJ, Lee S, Kim A, Kim Y, Park WY, et al. Cytoplasmic TrkA expression as a screen for detecting NTRK1 fusions in colorectal cancer. Transl Oncol. 2018; 11:764–70.
Article24. Hechtman JF, Benayed R, Hyman DM, Drilon A, Zehir A, Frosina D, et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am J Surg Pathol. 2017; 41:1547–51.
Article25. Mauri G, Valtorta E, Cerea G, Amatu A, Schirru M, Marrapese G, et al. TRKA expression and NTRK1 gene copy number across solid tumours. J Clin Pathol. 2018; 71:926–31.
Article26. Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsague X, Laporte L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014; 15:1319–31.
Article27. Nevens D, Nuyts S. HPV-positive head and neck tumours, a distinct clinical entity. B-ENT. 2015; 11:81–7.28. Lee SJ, Li GG, Kim ST, Hong ME, Jang J, Yoon N, et al. NTRK1 rearrangement in colorectal cancer patients: evidence for actionable target using patient-derived tumor cell line. Oncotarget. 2015; 6:39028–35.
Article29. Feller L, Wood NH, Khammissa RA, Lemmer J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 2: Human papillomavirus associated oral and oropharyngeal squamous cell carcinoma. Head Face Med. 2010; 6:15.
Article30. Shan C, Wei J, Hou R, Wu B, Yang Z, Wang L, et al. Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol Rep. 2016; 35:427–35.
Article31. Sclabas GM, Fujioka S, Schmidt C, Li Z, Frederick WA, Yang W, et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005; 11(2 Pt 1):440–9.32. Lee J, Jiffar T, Kupferman ME. A novel role for BDNF-TrkB in the regulation of chemotherapy resistance in head and neck squamous cell carcinoma. PLoS One. 2012; 7:e30246.
Article33. Yilmaz T, Jiffar T, de la Garza G, Lin H, Milas Z, Takahashi Y, et al. Theraputic targeting of Trk supresses tumor proliferation and enhances cisplatin activity in HNSCC. Cancer Biol Ther. 2010; 10:644–53.34. Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005; 105:4429–36.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synchronous Verrucous Carcinoma and Squamous Cell Carcinoma of Penis by Different Human Papillomavirus Infections
- Prognostic implications of stromal hyaluronic acid protein expression in resected oropharyngeal and oral cavity cancers
- Mutation of p53 Gene and Infection of Human Papillomavirus in Oral and Oropharyngeal Carcinoma
- Incidental finding of an extensive oropharyngeal mass in magnetic resonance imaging of a patient with temporomandibular disorder: A case report
- Role of Hepatocyte Growth Factor and c-met Gene Expression in Oral Cavity and Oropharyngeal Squamous Cell Carcinoma