Nutr Res Pract.
2019 Jun;13(3):196-204. 10.4162/nrp.2019.13.3.196.
Free fatty acid-induced histone acetyltransferase activity accelerates lipid accumulation in HepG2 cells
- Affiliations
-
- 1Korea Food Research Institute, 245, Nongsaengmyeong-ro, Iseo-myeon, Wanju-gun, Jeonbuk 55365, Republic of Korea. chkyoung@kfri.re.kr
- 2Department of Food Biotechnology, Korea University of Science & Technology, Daejeon 34113, Republic of Korea.
- KMID: 2453283
- DOI: http://doi.org/10.4162/nrp.2019.13.3.196
Abstract
- BACKGROUND/OBJECTIVES
Non-alcoholic fatty liver disease (NAFLD) is a common metabolic disease triggered by epigenetic alterations, including lysine acetylation at histone or non-histone proteins, affecting the stability or transcription of lipogenic genes. Although various natural dietary compounds have anti-lipogenic effects, their effects on the acetylation status and lipid metabolism in the liver have not been thoroughly investigated.
MATERIALS/METHODS
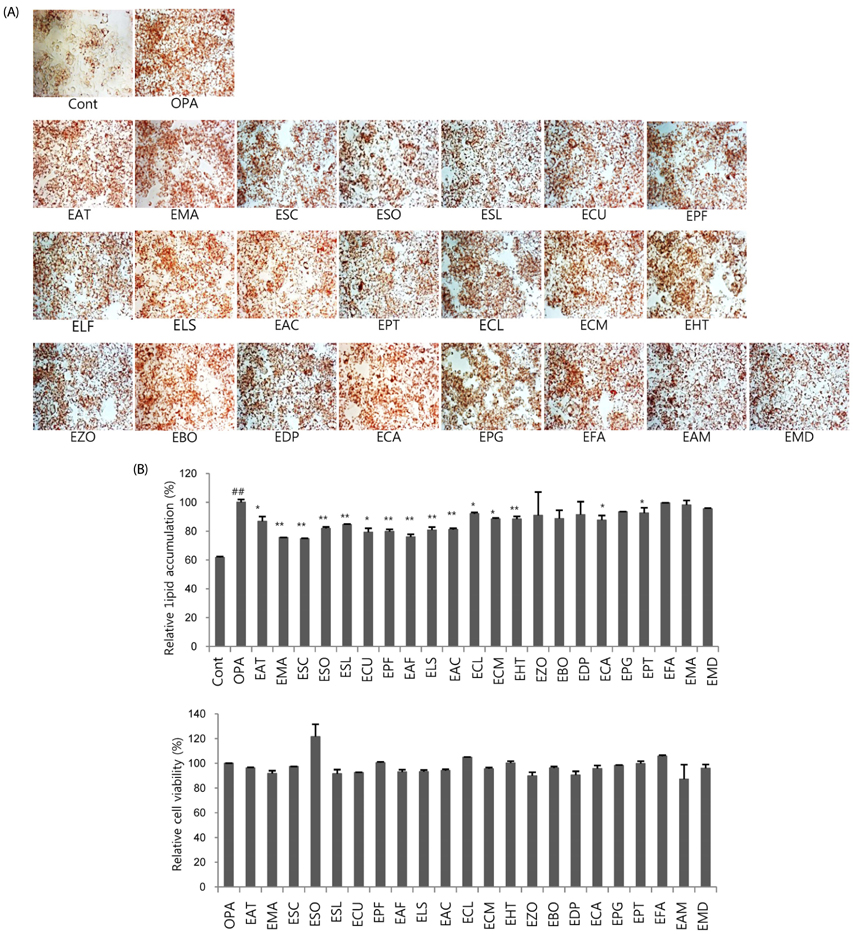
Following oleic-palmitic acid (OPA)-induced lipid accumulation in HepG2 cells, the acetylation status of histone and non-histone proteins, HAT activity, and mRNA expression of representative lipogenic genes, including PPARγ, SREBP-1c, ACLY, and FASN, were evaluated. Furthermore, correlations between lipid accumulation and HAT activity for 22 representative natural food extracts (NExs) were evaluated.
RESULTS
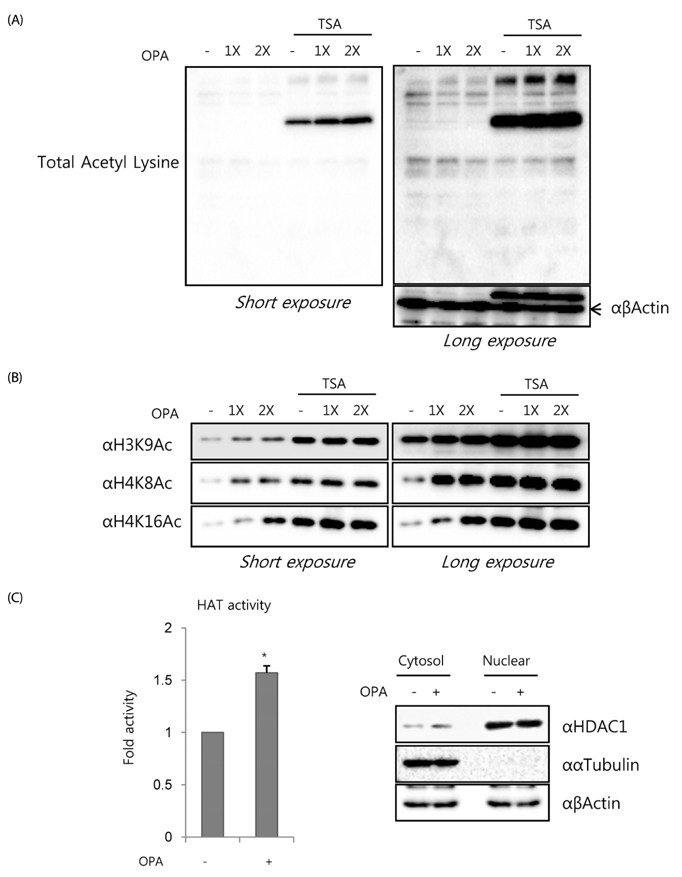
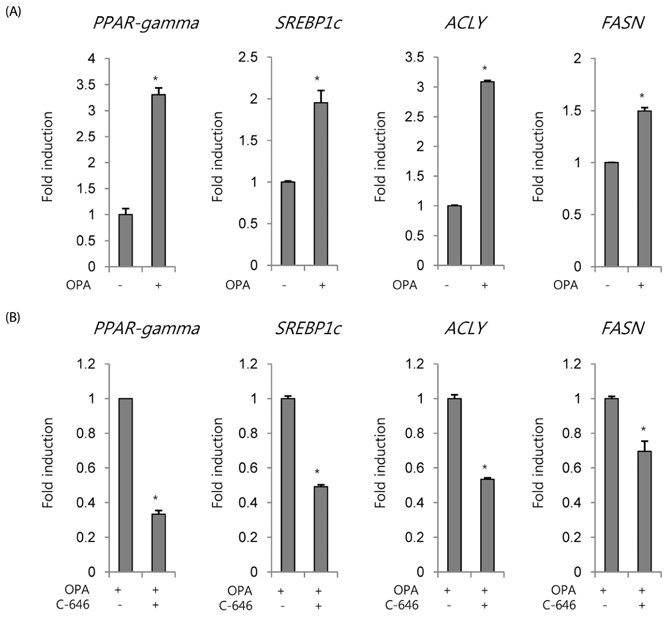
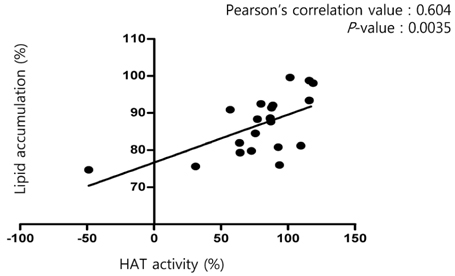
Non-histone protein acetylation increased following OPA treatment and the acetylation of histones H3K9, H4K8, and H4K16 was accelerated, accompanied by an increase in HAT activity. OPA-induced increases in the mRNA expression of lipogenic genes were down-regulated by C-646, a p300/CBP-specific inhibitor. Finally, we detected a positive correlation between HAT activity and lipid accumulation (Pearson's correlation coefficient = 0.604) using 22 NExs.
CONCLUSIONS
Our results suggest that NExs have novel applications as nutraceutical agents with HAT inhibitor activity for the prevention and treatment of NAFLD.
Keyword
MeSH Terms
-
Acetylation
Dietary Supplements
Epigenomics
Hep G2 Cells*
Histone Acetyltransferases*
Histones*
Lipid Metabolism
Lipogenesis
Liver
Lysine
Metabolic Diseases
Non-alcoholic Fatty Liver Disease
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Histone Acetyltransferases
Histones
Lysine
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Figure
Reference
-
1. Ahmed MH, Husain NE, Almobarak AO. Nonalcoholic Fatty liver disease and risk of diabetes and cardiovascular disease: what is important for primary care physicians? J Family Med Prim Care. 2015; 4:45–52.
Article2. Sun C, Fan JG, Qiao L. Potential epigenetic mechanism in non-alcoholic fatty liver disease. Int J Mol Sci. 2015; 16:5161–5179.3. Wang Q, Guo S, Gao Y. Protein lysine acetylated/deacetylated enzymes and the metabolism-related diseases. Adv Biosci Biotechnol. 2016; 7:454–467.
Article4. Castaño-Cerezo S, Bernal V, Post H, Fuhrer T, Cappadona S, Sánchez-Díaz NC, Sauer U, Heck AJ, Altelaar AF, Cánovas M. Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol Syst Biol. 2014; 10:762.
Article5. van Noort V, Seebacher J, Bader S, Mohammed S, Vonkova I, Betts MJ, Kühner S, Kumar R, Maier T, O'Flaherty M, Rybin V, Schmeisky A, Yus E, Stülke J, Serrano L, Russell RB, Heck AJ, Bork P, Gavin AC. Cross-talk between phosphorylation and lysine acetylation in a genome-reduced bacterium. Mol Syst Biol. 2012; 8:571.
Article6. Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli. J Microbiol Biotechnol. 2008; 18:1529–1536.7. Kim D, Yu BJ, Kim JA, Lee YJ, Choi SG, Kang S, Pan JG. The acetylproteome of Gram-positive model bacterium Bacillus subtilis. Proteomics. 2013; 13:1726–1736.
Article8. Fukushima A, Lopaschuk GD. Acetylation control of cardiac fatty acid β-oxidation and energy metabolism in obesity, diabetes, and heart failure. Biochim Biophys Acta. 2016; 1862:2211–2220.
Article9. LaBarge S, Migdal C, Schenk S. Is acetylation a metabolic rheostat that regulates skeletal muscle insulin action? Mol Cells. 2015; 38:297–303.
Article10. Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006; 23:289–296.
Article11. Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008; 9:206–218.
Article12. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009; 10:32–42.
Article13. Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010; 327:1000–1004.
Article14. Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010; 327:1004–1007.
Article15. Podrini C, Borghesan M, Greco A, Pazienza V, Mazzoccoli G, Vinciguerra M. Redox homeostasis and epigenetics in non-alcoholic fatty liver disease (NAFLD). Curr Pharm Des. 2013; 19:2737–2746.
Article16. Xu X, So JS, Park JG, Lee AH. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis. 2013; 33:301–311.
Article17. Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010; 120:4316–4331.
Article18. Shankar E, Kanwal R, Candamo M, Gupta S. Dietary phytochemicals as epigenetic modifiers in cancer: promise and challenges. Semin Cancer Biol. 2016; 40-41:82–99.
Article19. Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, Taylor KD, Wilson L, Cummings OW, Chen YD, Rotter JI. Nonalcoholic Steatohepatitis Clinical Research Network. Genomewide association study identifies variants associated with histologic features of nonalcoholic Fatty liver disease. Gastroenterology. 2010; 139:1567–1576. 1576.e1–1576.e6.
Article20. Hwang JT, Shin EJ, Chung MY, Park JH, Chung S, Choi HK. Ethanol extract of Allium fistulosum inhibits development of non-alcoholic fatty liver disease. Nutr Res Pract. 2018; 12:110–117.
Article21. Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, Lee JM, Yoon HG. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009; 69:583–592.
Article22. Pan MH, Lai CS, Tsai ML, Ho CT. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol Nutr Food Res. 2014; 58:147–171.
Article23. Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, Martino JS, Castaño GO, Sookoian S. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 2013; 62:1356–1363.
Article24. Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013; 10:645–655.
Article25. Afonso MB, Rodrigues PM, Simão AL, Castro RE. Circulating microRNAs as potential biomarkers in non-alcoholic fatty liver disease and hepatocellular carcinoma. J Clin Med. 2016; 5:30.
Article26. Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008; 22:3549–3560.
Article27. Tian Y, Wong VW, Chan HL, Cheng AS. Epigenetic regulation of hepatocellular carcinoma in non-alcoholic fatty liver disease. Semin Cancer Biol. 2013; 23:471–482.
Article28. Lo KA, Bauchmann MK, Baumann AP, Donahue CJ, Thiede MA, Hayes LS, des Etages SA, Fraenkel E. Genome-wide profiling of H3K56 acetylation and transcription factor binding sites in human adipocytes. PLoS One. 2011; 6:e19778.
Article29. Mikula M, Majewska A, Ledwon JK, Dzwonek A, Ostrowski J. Obesity increases histone H3 lysine 9 and 18 acetylation at Tnfa and Ccl2 genes in mouse liver. Int J Mol Med. 2014; 34:1647–1654.
Article30. Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res. 2014; 103:485–497.
Article31. Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010; 285:33959–33970.
Article32. Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000; 64:435–459.
Article33. Verdone L, Agricola E, Caserta M, Di Mauro E. Histone acetylation in gene regulation. Brief Funct Genomics Proteomics. 2006; 5:209–221.
Article34. Kurdistani SK, Grunstein M. Histone acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol. 2003; 4:276–284.
Article35. Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999; 19:5495–5503.
Article36. Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011; 96:1424–1430.
Article37. Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Ther Clin Risk Manag. 2007; 3:1153–1163.38. Rodgers RJ, Tschöp MH, Wilding JP. Anti-obesity drugs: past, present and future. Dis Model Mech. 2012; 5:621–626.
Article39. Ann JY, Eo H, Lim Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015; 10:46.
Article40. Sun JH, Liu X, Cong LX, Li H, Zhang CY, Chen JG, Wang CM. Metabolomics study of the therapeutic mechanism of Schisandra Chinensis lignans in diet-induced hyperlipidemia mice. Lipids Health Dis. 2017; 16:145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Doxorubicin Attenuates Free Fatty Acid-Induced Lipid Accumulation via Stimulation of p53 in HepG2 Cells
- Downregulation of GNAI3 Promotes the Pathogenesis of Methionine/Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease
- Picroside II attenuates fatty acid accumulation in HepG2 cells via modulation of fatty acid uptake and synthesis
- Physiologic and epigenetic effects of nutrients on disease pathways
- Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway