Yonsei Med J.
2019 Aug;60(8):713-719. 10.3349/ymj.2019.60.8.713.
A Disintegrin and Metalloproteinase 8 as a Potential Blood Biomarker for Early Diagnosis of Gastric Cancer
- Affiliations
-
- 1Department of Laboratory Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jlim@yuhs.ac
- KMID: 2452951
- DOI: http://doi.org/10.3349/ymj.2019.60.8.713
Abstract
- PURPOSE
We aimed to evaluate the clinical significance of a disintegrin and metalloproteinase 8 (ADAM 8) as a potential blood biomarker for gastric cancer (GC).
MATERIALS AND METHODS
Blood ADAM 8 was measured by ELISA. Cytokines/chemokines [interleukin-23 (IL-23), stromal cell-derived factor 1α/CXC chemokine ligand 12 (SDF-1α/CXCL12), interleukin-8 (IL-8), and soluble CD40 ligand (sCD40L)] were measured by chemiluminescent immunoassay. They were compared among five groups; normal/gastritis, high-risk, early GC (EGC), advanced GC (AGC) without distant metastasis, and AGC with distant metastasis by one-way analysis of variance in both training (n=80) and validation dataset (n=241). Clinicopathological features of GC and GC-associated cytokines were evaluated for their correlations with blood ADAM 8. To evaluate the diagnostic accuracy to predict GC, receiver operating characteristic (ROC) curve and logistic regression were used.
RESULTS
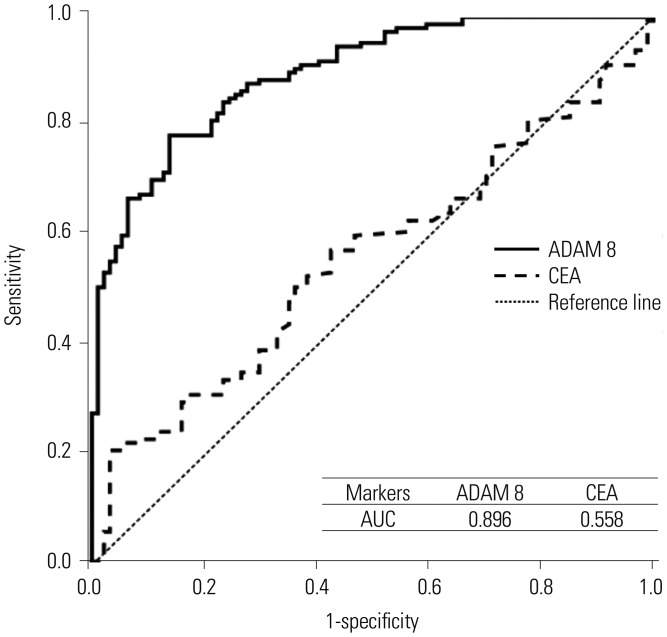
Blood ADAM 8 significantly increased along GC carcinogenesis in both training (ANOVA, p<0.001) and validation dataset (p<0.001). It was significantly higher in EGC compared to high-risk (post-hoc Bonferroni, p=0.041) and normal (p<0.001). It was also higher in AGC compared with high-risk (p<0.001) and normal (p<0.001) groups. However, no significant difference was found between cancer groups. Blood ADAM 8 was correlated with N-stage (Spearman's correlation, γs=0.320, p=0.011), but not with T-stage or M-stage. Pearson's correlations showed blood ADAM 8 was closely correlated with pre-inflammatory cytokines, IL-23 (p=0.036) and SDF-1α/CXCL12 (p=0.037); however, it was not correlated with pro-angiogenic cytokine IL-8 (p=0.313), and sCD40L (p=0.702). ROC curve and logistic regression demonstrated that blood ADAM 8 showed higher diagnostic accuracy (sensitivity, 73.7%; specificity, 86.2%) than CEA (sensitivity, 23.1%; specificity, 91.4%). Combination of ADAM 8 and CEA further increased the diagnostic accuracy to predict GC (sensitivity, 81.8%; specificity, 84.0%).
CONCLUSION
Blood ADAM 8 is a promising biomarker for early detection of GC.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010; 19:1893–1907. PMID: 20647400.
Article2. Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005; 9:718–725. PMID: 15862270.
Article3. Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011; 14:301–316. PMID: 21894577.
Article4. Karimi P, Islami F, Anandasabapathy S, Freedman N, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014; 23:700–713. PMID: 24618998.
Article5. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014; 17:26–33. PMID: 23572188.
Article6. Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, Varthalitis J, et al. CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol. 1997; 20:348–353. PMID: 9256887.
Article7. Fan B, Xiong B. Investigation of serum tumor markers in the diagnosis of gastric cancer. Hepatogastroenterology. 2011; 58:239–245. PMID: 21510322.8. Duffy MJ, McKiernan E, O'Donovan N, McGowan PM. Role of ADAMs in cancer formation and progression. Clin Cancer Res. 2009; 15:1140–1144. PMID: 19228719.
Article9. Aydin D, Bilici A, Yavuzer D, Kefeli U, Tan A, Ercelep O, et al. Prognostic significance of ADAM17 expression in patients with gastric cancer who underwent curative gastrectomy. Clin Transl Oncol. 2015; 17:604–611. PMID: 25786367.
Article10. Chen J, Chen X, Wang F, Gao H, Hu W. Dihydroartemisinin suppresses glioma proliferation and invasion via inhibition of the ADAM17 pathway. Neurol Sci. 2015; 36:435–440. PMID: 25301262.
Article11. Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007; 98:621–628. PMID: 17355265.
Article12. Mullooly M, McGowan PM, Crown J, Duffy MJ. The ADAMs family of proteases as targets for the treatment of Cancer. Cancer Biol Ther. 2016; 17:870–880. PMID: 27115328.
Article13. Huang J, Bai Y, Huo L, Xiao J, Fan X, Yang Z, et al. Upregulation of a disintegrin and metalloprotease 8 is associated with progression and prognosis of patients with gastric cancer. Transl Res. 2015; 166:602–613. PMID: 26024798.
Article14. Tong W, Ye F, He L, Cui L, Cui M, Hu Y, et al. Serum biomarker panels for diagnosis of gastric cancer. Onco Targets Ther. 2016; 9:2455–2463. PMID: 27217769.15. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992; 52:6735–6740. PMID: 1458460.16. Reim D, Loos M, Vogl F, Novotny A, Schuster T, Langer R, et al. Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. J Clin Oncol. 2013; 31:263–271. PMID: 23213098.
Article17. Koller G, Schlomann U, Golfi P, Ferdous T, Naus S, Bartsch JW. ADAM 8/MS2/CD156, an emerging drug target in the treatment of inflammatory and invasive pathologies. Curr Pharm Des. 2009; 15:2272–2281. PMID: 19601829.18. Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, et al. The metalloprotease disintegrin ADAM 8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002; 277:48210–48219. PMID: 12372841.19. Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM 8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003; 278:30469–30477. PMID: 12777399.20. Li W, Ye F, Wang D, Sun X, Tong W, Lian G, et al. Protein predictive signatures for lymph node metastasis of gastric cancer. Int J Cancer. 2013; 132:1851–1859. PMID: 23011604.
Article21. Romagnoli M, Mineva ND, Polmear M, Conrad C, Srinivasan S, Loussouarn D, et al. ADAM 8 expression in invasive breast cancer promotes tumor dissemination and metastasis. EMBO Mol Med. 2014; 6:278–294. PMID: 24375628.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liquid Biopsy: An Emerging Diagnostic, Prognostic, and Predictive Tool in Gastric Cancer
- Screening of gastric cancer
- Molecular Pathology of Gastric Cancer
- Potential of the Microbiome as a Biomarker for Early Diagnosis and Prognosis of Breast Cancer
- The Role of Serum Pepsinogen in the Detection of Gastric Cancer