Nat Prod Sci.
2019 Jun;25(2):130-135. 10.20307/nps.2019.25.2.130.
Chemical Constituents of Impatiens balsamina Stems and Their Biological Activities
- Affiliations
-
- 1Natural Products Laboratory, School of Pharmacy, Sungkyunkwan University, Suwon 16419, Korea. krlee@skku.ac.kr
- 2Gachon Institute of Parmaceutical Science, Gachon University, 191 Hambakmoero, Yeonsu-gu, Incheon 21936, Republic of Korea.
- 3College of pharmacy, Gachon University, 191 Hambakmoero, Yeonsu-gu, Incheon 21936, Republic of Korea.
- KMID: 2452782
- DOI: http://doi.org/10.20307/nps.2019.25.2.130
Abstract
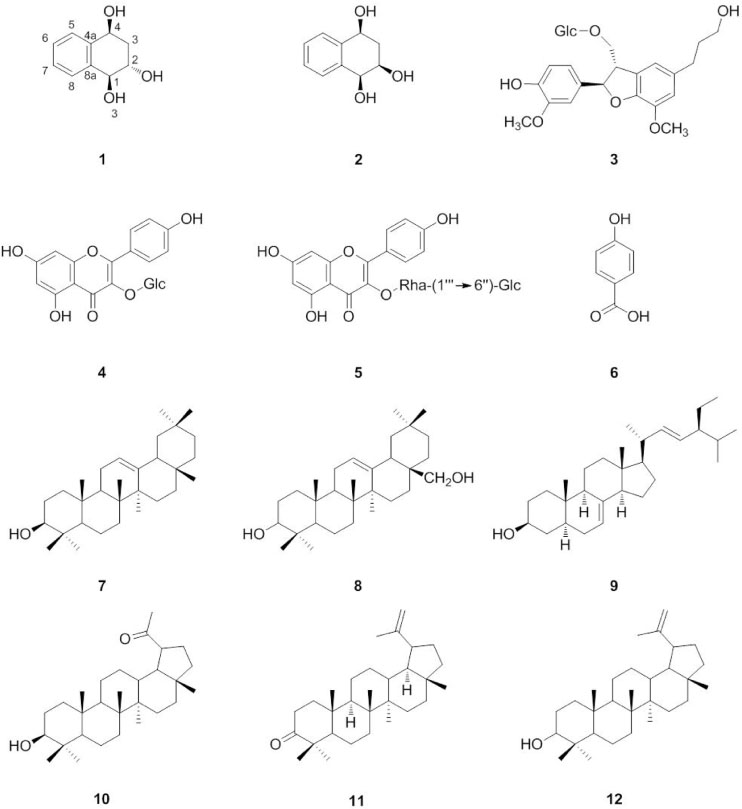
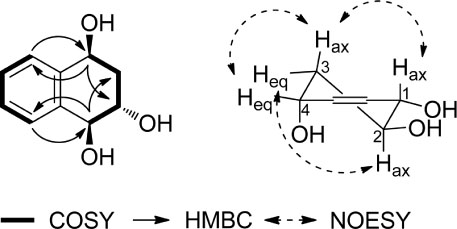
- The purification of the MeOH extract from Impatiens balsamina by repeated column chromatography led to the isolation of one new tetrahydronaphthalene (1), together with eleven known compounds (2 - 12). The structure of the new compound (1) was determined by spectral data analysis (1H and 13C-NMR, 1H-1H COSY, HSQC, HMBC, NOESY, and HR-ESI-MS). Isolated compounds (1 - 12) were evaluated for their inhibitory effects on NO production in LPS-activated murine microglial BV-2 cells and their effects on NGF secretion from C6 glioma cells. Compounds 3, 7, and 10 reduced NO levels in LPS-activated murine microglial cells with IC50 values of 26.89, 25.59, and 44.21 µM, respectively. Compounds 1, 5, and 9 upregulated NGF secretion to 153.09 ± 4.66, 156.88 ± 8.86, and 157.34 ± 3.30%, respectively.
MeSH Terms
Figure
Reference
-
1. Meenu B, Neeraja ED, Rejimon G, Varghese A. J Chem Pharm Res. 2015; 7:16–21.2. Ding ZS, Jiang FS, Chen NP, Lv GY, Zhu CG. Molecules. 2008; 13:220–229.3. Kang SN, Goo YM, Yang MR, Ibrahim RIH, Cho JH, Kim IS, Lee OH. Molecules. 2013; 18:6356–6365.4. Shoji N, Umeyama A, Yosikawa K, Nagai M, Arihara S. Phytochemistry. 1994; 37:1437–1441.5. Panichayupakaranant P, Noguchi H, De-Eknamkul W, Sankawa U. Phytochemistry. 1995; 40:1141–1143.6. Li Q, Zhang X, Cao J, Guo Z, Lou Y, Ding M, Zhao Y. Fitoterapia. 2015; 105:234–239.7. Ishiguro K, Ohira Y, Oku H. J Nat Prod. 1998; 61:1126–1129.8. Imam MZ, Nahar N, Akter S, Rana MS. J Ethnopharmacol. 2012; 142:804–810.9. Kim CS, Subedi L, Kim SY, Choi SU, Choi SJ, Son MW, Kim KH, Lee KR. Phytochem lett. 2015; 14:215–220.10. Kim CS, Bae M, O J, Subedi L, Suh WS, Choi SZ, Son MW, Kim SY, Choi SU, Oh DC, Lee KR. J Nat Prod. 2017; 80:471–478.11. Rashid A, Read G. J Chem Soc. 1969; 15:2053–2058.12. Zhao XC, Du JL, Xie YG, Zhang Y, Jin HZ. Chem Nat Compd. 2018; 54:556–558.13. Jayasinghe ULB, Balasooriya BAIS, Bandara AGD, Fujimoto Y. Nat Prod Res. 2004; 18:499–502.14. Park SY, Kim SJ, Lee SY, Bae KH, Kang SS. Nat Prod Sci. 2008; 14:281–288.15. Rho T, Yoon KD. Nat Prod Sci. 2017; 23:253–257.16. Wang H, Tian X, Chen YZ. J Chin Chem Soc. 2002; 49:433–436.17. Ge YC, Zhang HJ, Lei JX, Wang KW. Chem Nat Compd. 2018; 54:600–602.18. Choi SZ, Lee SO, Choi SU, Lee KR. Arch Pharm Res. 2003; 26:521–525.19. Kotowicz C, Catalan CAN, Griffin CL, Herz W. Biochem Syst Ecol. 2005; 33:737–742.20. Puapairoj P, Naengchomnong W, Kijjoa A, Pinto MM, Pedro M, Nascimento MS, Silva AM, Herz W. Planta Med. 2005; 71:208–213.21. Fotie J, Bohle DS, Leimanis ML, Georges E, Rukunga G, Nkengfack AE. J Nat Prod. 2006; 69:62–67.22. Chen XM, Qian SH, Feng F. Chine Chem Lett. 2010; 21:440–442.23. Naemura K, Wakebe T, Hirose K, Tobe Y. Tetrahedron Asymmetry. 1997; 15:2585–2595.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasmopara elegantissima sp. nov. (Oomycota, Peronosporales), a Downy Mildew Species Specialized to Impatiens textori (Balsaminaceae)
- Essential Oil of Marrubium vulgare: Chemical Composition and Biological Activities. A Review
- WebChemDB: An Integrated Chemical Database Retrieval System
- A New Dimeric Lignan from the Stems of Willughbeia edulis
- Biological Activity of Chemical Constituents Isolated from Strain Chlamydomonas sp. KSF108 (Chlamydomonadaceae)