Ann Dermatol.
2019 Aug;31(4):393-402. 10.5021/ad.2019.31.4.393.
Increased Circulating CXCL10 in Non-Segmental Vitiligo Concomitant with Autoimmune Thyroid Disease and Alopecia Areata
- Affiliations
-
- 1Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China. flora_xiang@vip.163.com, e3dangdang@hotmail.com
- KMID: 2451476
- DOI: http://doi.org/10.5021/ad.2019.31.4.393
Abstract
- BACKGROUND
Vitiligo is a common acquired pigmentary disease caused by destruction of epidermal melanocytes in underlying autoimmune response. Few studies have been focused on the role of chemokines in non-segmental vitiligo (NSV) concomitant with autoimmune thyroid disease (AITD) and alopecia areata (AA).
OBJECTIVE
The aim of this study was to determine the best serum biomarker for predictive role in the progression of vitiligo and to evaluate the influence of AA and/or AITD on vitiligo by using the biomarker.
METHODS
This prospective cohort study recruited 45 NSV patients: 14 without either AITD or AA, 12 with AITD, 11 with AA, and 8 with both AITD and AA. Serum levels of CXCL1, CXCL8, CXCL9, CXCL10, CXCL12, CXCL13, and CXCL16 were analyzed by ELISA. CXCR3 mRNA expression was detected on PBMCs by RT-PCR. Improvement was evaluated using repigmentation scales.
RESULTS
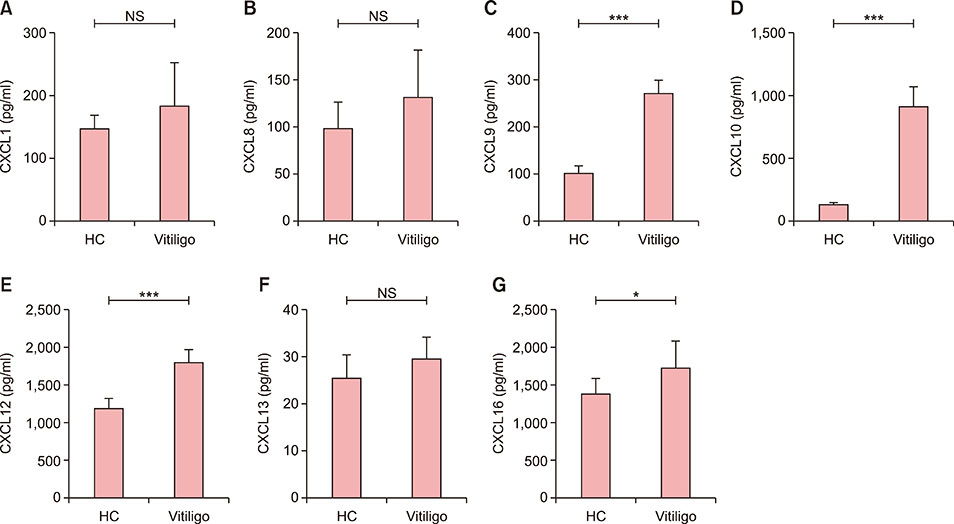
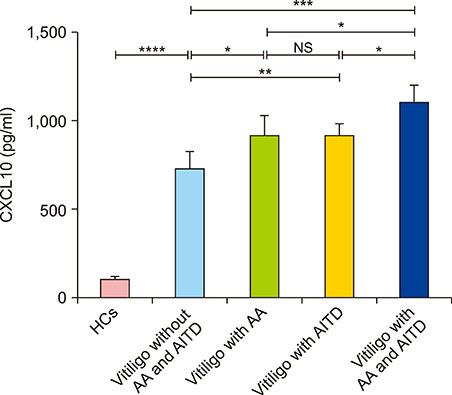
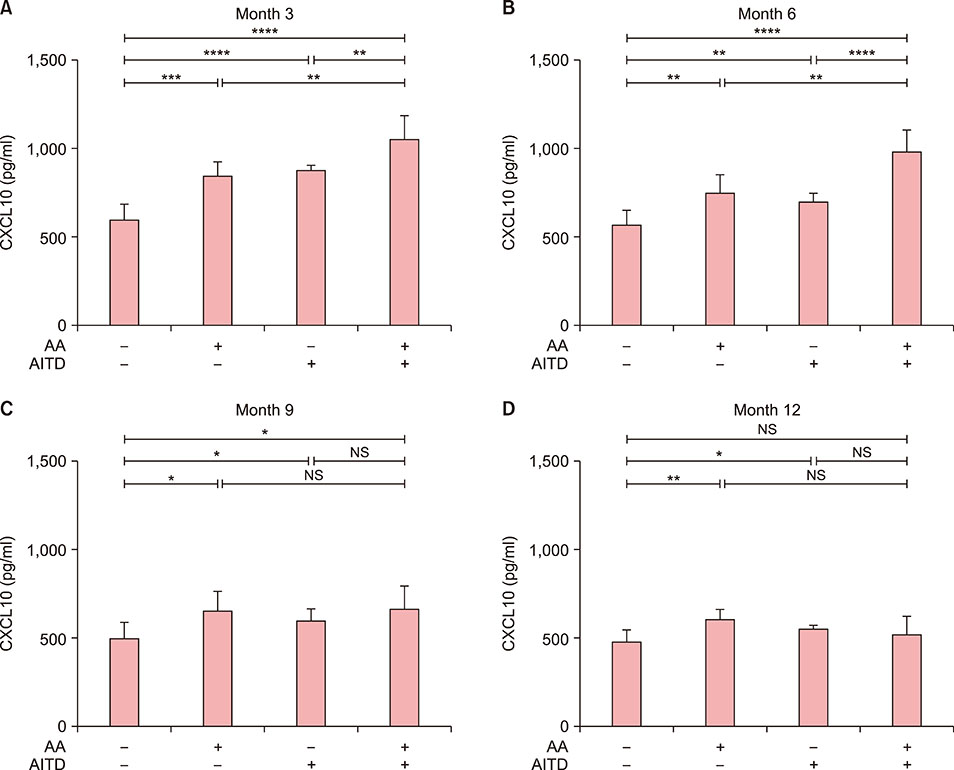
Serum CXCL10 levels, along with the expression of CXCR3 mRNA were higher in NSV patients with AITD or AA alone than in those without AITD or AA. Moreover, serum CXCL10 levels, along with the expression of CXCR3 mRNA were higher in NSV patients with both AITD and AA than in those with AITD or AA alone. Poorer repigmentation was observed in NSV patients with both AA and AITD than in those with AA or AITD alone.
CONCLUSION
CXCL10 could be a biomarker to predict the progression of NSV. Dermatologists should pay much attention to those NSV patients concomitant with AITD and/or AA, for comorbidity might lead to more active autoimmune reaction.
MeSH Terms
Figure
Reference
-
1. Picardo M, Dell'Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, et al. Vitiligo. Nat Rev Dis Primers. 2015; 1:15011.
Article2. Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol Rev. 2016; 269:11–25.
Article3. Rork JF, Rashighi M, Harris JE. Understanding autoimmunity of vitiligo and alopecia areata. Curr Opin Pediatr. 2016; 28:463–469.
Article4. Liu M, Murphy E, Amerson EH. Rethinking screening for thyroid autoimmunity in vitiligo. J Am Acad Dermatol. 2016; 75:1278–1280.
Article5. Mockenhaupt M, Peters F, Schwenk-Davoine I, Herouy Y, Schraufstätter I, Elsner P, et al. Evidence of involvement of CXC-chemokines in proliferation of cultivated human melanocytes. Int J Mol Med. 2003; 12:597–601.
Article6. Miniati A, Weng Z, Zhang B, Therianou A, Vasiadi M, Nicolaidou E, et al. Stimulated human melanocytes express and release interleukin-8, which is inhibited by luteolin: relevance to early vitiligo. Clin Exp Dermatol. 2014; 39:54–57.
Article7. Lee HT, Shiao YM, Wu TH, Chen WS, Hsu YH, Tsai SF, et al. Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol. 2010; 37:45–52.
Article8. Wutte N, Kovacs G, Berghold A, Reiter H, Aberer W, Aberer E. CXCL13 and B-cell activating factor as putative biomarkers in systemic sclerosis. Br J Dermatol. 2013; 169:723–725.
Article9. Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014; 6:223ra23.
Article10. Wang XX, Wang QQ, Wu JQ, Jiang M, Chen L, Zhang CF, et al. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016; 174:1318–1326.
Article11. Speeckaert R, Voet S, Hoste E, van Geel N. S100B is a potential disease activity marker in nonsegmental vitiligo. J Invest Dermatol. 2017; 137:1445–1453.
Article12. Kim JY, Lee EJ, Seo J, Oh SH. Impact of high-mobility group box 1 on melanocytic survival and its involvement in the pathogenesis of vitiligo. Br J Dermatol. 2017; 176:1558–1568.
Article13. Speeckaert R, Ongenae K, van Geel N. Alterations of CXCL12 in serum of patients with vitiligo. J Invest Dermatol. 2017; 137:1586–1588.
Article14. Li S, Zhu G, Yang Y, Jian Z, Guo S, Dai W, et al. Oxidative stress drives CD8+ T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J Allergy Clin Immunol. 2017; 140:177–189.e9.15. Stinco G, Buligan C, Grimaldi F, Valent F, Patrone P. Serological screening for autoimmune polyendocrine syndromes in patients with vitiligo. J Eur Acad Dermatol Venereol. 2012; 26:1041–1042.
Article16. Laberge G, Mailloux CM, Gowan K, Holland P, Bennett DC, Fain PR, et al. Early disease onset and increased risk of other autoimmune diseases in familial generalized vitiligo. Pigment Cell Res. 2005; 18:300–305.
Article17. Vrijman C, Kroon MW, Limpens J, Leeflang MM, Luiten RM, van der Veen JP, et al. The prevalence of thyroid disease in patients with vitiligo: a systematic review. Br J Dermatol. 2012; 167:1224–1235.
Article18. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014; 20:1043–1049.
Article19. Taïeb A, Picardo M. VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007; 20:27–35.
Article20. Fröhlich E, Wahl R. Thyroid autoimmunity: role of antithyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017; 8:521.
Article21. Maouia A, Sormani L, Youssef M, Helal AN, Kassab A, Passeron T. Differential expression of CXCL9, CXCL10, and IFN-γ in vitiligo and alopecia areata patients. Pigment Cell Melanoma Res. 2017; 30:259–261.
Article22. Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, et al. Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J Invest Dermatol. 2017; 137:350–358.
Article23. Campbell JD, Gangur V, Simons FE, HayGlass KT. Allergic humans are hyporesponsive to a CXCR3 ligand-mediated Th1 immunity-promoting loop. FASEB J. 2004; 18:329–331.24. Lang KS, Caroli CC, Muhm A, Wernet D, Moris A, Schittek B, et al. HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol. 2001; 116:891–897.
Article25. van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009; 129:2220–2232.
Article26. Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009; 19:495–501.
Article27. McElwee KJ, Gilhar A, Tobin DJ, Ramot Y, Sundberg JP, Nakamura M, et al. What causes alopecia areata? Exp Dermatol. 2013; 22:609–626.
Article28. Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007; 117:2019–2027.
Article29. Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J Am Acad Dermatol. 2016; 74:295–302.
Article30. Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014; 10:e1004123.31. Spritz RA, Gowan K, Bennett DC, Fain PR. Novel vitiligo susceptibility loci on chromosomes 7 (AIS2) and 8 (AIS3), confirmation of SLEV1 on chromosome 17, and their roles in an autoimmune diathesis. Am J Hum Genet. 2004; 74:188–191.
Article32. Gey A, Diallo A, Seneschal J, Léauté-Labrèze C, Boralevi F, Jouary T, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013; 168:756–761.
Article33. Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007; 28:492–520.
Article34. Ferrari SM, Fallahi P, Santaguida G, Virili C, Ruffilli I, Ragusa F, et al. Circulating CXCL10 is increased in non-segmental vitiligo, in presence or absence of autoimmune thyroiditis. Autoimmun Rev. 2017; 16:946–950.
Article35. Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015; 26:311–327.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Colocalization of Segmental Vitiligo and Alopecia Areata
- A Case of Alopecia Areata Associated with Autoimmune Polyglandular Syndrome Type III
- A Case of Alopecia Areata Associated with Myasthenia Gravis and Thymoma
- Comorbid Autoimmune Diseases among Patients with Alopecia Areata: A Population-based Case-control Study in Korea
- A Study on the Frequency of the Autoimmune Disorders in Vitiligo Patients