J Clin Neurol.
2019 Jan;15(1):46-53. 10.3988/jcn.2019.15.1.46.
White-Matter Hyperintensities and Lacunar Infarcts Are Associated with an Increased Risk of Alzheimer's Disease in the Elderly in China
- Affiliations
-
- 1Battalion 3 of Cadet Brigade, Third Military Medical University (Army Medical University), Chongqing, China.
- 2Department of Neurology, Daping Hospital, Third Military Medical University (Army Medical University), Chongqing, China. zhouhuad@163.com, taoyong8987@sina.com
- 3Postgraduate School, Bengbu Medical College, Anhui, China.
- 4Rashid Laboratory for Developmental Neurobiology, Department of Psychiatry and Behavioral Neurosciences, Morsani College of Medicine, University of South Florida, Tampa, FL, USA.
- 5Department of Neurology, the People's Hospital of Banan District, Chongqing, China.
- 6Department of Epidemiology, College of Preventive Medicine, Third Military Medical University (Army Medical University), Chongqing, China.
- 7Department of Neurology, Qianjiang National Hospital, Chongqing, China.
- KMID: 2451144
- DOI: http://doi.org/10.3988/jcn.2019.15.1.46
Abstract
- BACKGROUND AND PURPOSE
This study investigated the contribution of white-matter hyperintensities (WMH) and lacunar infarcts (LI) to the risk of Alzheimer's disease (AD) in an elderly cohort in China.
METHODS
Older adults who were initially cognitively normal were examined with MRI at baseline, and followed for 5 years. WMH were classified as mild, moderate, or severe, and LI were classified into a few LI (1 to 3) or many LI (≥4). Cognitive function was assessed using the Mini Mental State Examination and the Activities of Daily Living scale.
RESULTS
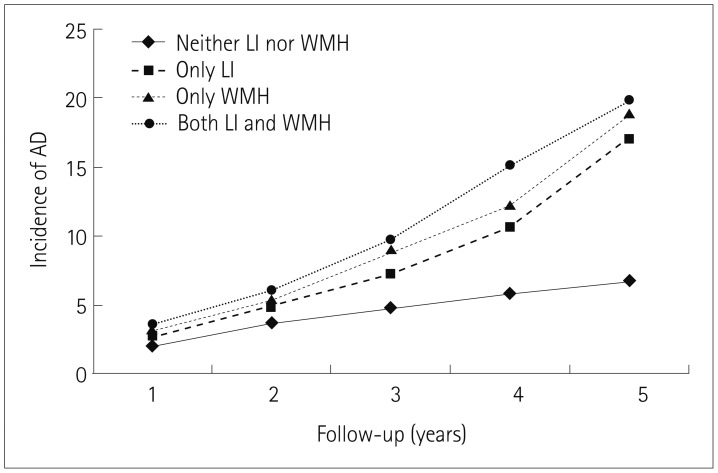
Among the 2,626 subjects, 357 developed AD by the end of the 5-year follow-up period. After adjusting for age and other potential confounders, having only WMH, having only LI, and having both WMH and LI were associated with an increased risk of developing AD compared with having neither WMH nor LI. Moderate and severe WMH were associated with an increased risk of developing AD compared with no WMH. Furthermore, patients with many LI had an increased risk of developing AD compared with no LI.
CONCLUSIONS
Having moderate or severe WMH and many LI were associated with an increased risk of developing AD, with this being particularly striking when both WMH and LI were present.
MeSH Terms
Figure
Reference
-
1. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015; 11:718–726. PMID: 26045020.
Article2. Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, et al. Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS One. 2014; 9:e87095. PMID: 24489845.
Article3. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012; 2012:367516. PMID: 23243502.
Article4. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014; 13:788–794. PMID: 25030513.
Article5. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011; 377:1019–1031. PMID: 21371747.
Article6. Scott JA, Braskie MN, Tosun D, Thompson PM, Weiner M, DeCarli C, et al. Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front Aging Neurosci. 2015; 7:221. PMID: 26648866.
Article7. Park JH, Ovbiagele B. Response to the comment ‘conflicting evidence on the association of white matter hyperintensities with large-artery disease’. Eur J Neurol. 2015; 22:e81. PMID: 26371442.
Article8. Nolze-Charron G, Mouiha A, Duchesne S, Bocti C. Alzheimer's Disease Neuroimaging Initiative. White matter hyperintensities in mild cognitive impairment and lower risk of cognitive decline. J Alzheimers Dis. 2015; 46:855–862. PMID: 26402625.
Article9. Altamura C, Scrascia F, Quattrocchi CC, Errante Y, Gangemi E, Curcio G, et al. Regional MRI diffusion, white-matter hyperintensities, and cognitive function in Alzheimer's disease and vascular dementia. J Clin Neurol. 2016; 12:201–208. PMID: 27074295.
Article10. Meguro K, Ishii H, Kasuya M, Akanuma K, Meguro M, Kasai M, et al. Incidence of dementia and associated risk factors in Japan: The Osaki-Tajiri Project. J Neurol Sci. 2007; 260:175–182. PMID: 17553526.
Article11. Zhang X, Ding L, Yuan J, Qin W, Hu W. Spatial relationship between acute lacunar infarction and white matter hyperintensities. Eur Neurol. 2015; 74:259–266. PMID: 26645081.
Article12. Zhou S, Zhou R, Zhong T, Li R, Tan J, Zhou H. Association of smoking and alcohol drinking with dementia risk among elderly men in China. Curr Alzheimer Res. 2014; 11:899–907. PMID: 25274108.
Article13. Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004; 251:421–427. PMID: 15083286.
Article14. Fuld PA. The fuld object-memory evaluation. Chicago: Stoelting Instrument Co.;1981.15. Zhang M, Qu G, Wang Z, Cai G, Katzman R, Simon D, et al. Prevalence study on dementia and Alzheimer disease. Zhonghua Yi Xue Za Zhi. 1990; 70:424–428. PMID: 2174278.16. Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1992; 49:448–452. PMID: 1580805.17. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975; 23:433–441. PMID: 1159263.
Article18. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23:56–62. PMID: 14399272.
Article19. Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006; 37:2220–2241. PMID: 16917086.
Article20. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269. PMID: 21514250.
Article21. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993; 43:250–260. PMID: 8094895.
Article22. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. Washington: American Psychiatric Association;2000.23. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356. PMID: 3496763.
Article24. van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005; 36:2116–2120. PMID: 16141425.25. Admiraal-Behloul F, van den Heuvel DM, Olofsen H, van Osch MJ, van der Grond J, van Buchem MA, et al. Fully automatic segmentation of white matter hyperintensities in MR images of the elderly. Neuroimage. 2005; 28:607–617. PMID: 16129626.
Article26. van der Flier WM, van den Heuvel DM, Weverling-Rijnsburger AW, Bollen EL, Westendorp RG, van Buchem MA, et al. Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann Neurol. 2002; 52:62–67. PMID: 12112048.
Article27. Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging. 2015; 36:27–32. PMID: 25155654.
Article28. Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003; 22:13–22. PMID: 12566949.
Article29. Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004; 61:1531–1534. PMID: 15477506.
Article30. Diaz JF, Merskey H, Hachinski VC, Lee DH, Boniferro M, Wong CJ, et al. Improved recognition of leukoaraiosis and cognitive impairment in Alzheimer's disease. Arch Neurol. 1991; 48:1022–1025. PMID: 1929892.
Article31. Kitagawa K, Miwa K, Yagita Y, Okazaki S, Sakaguchi M, Mochizuki H. Association between carotid stenosis or lacunar infarction and incident dementia in patients with vascular risk factors. Eur J Neurol. 2015; 22:187–192. PMID: 25164480.
Article32. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003; 348:1215–1222. PMID: 12660385.
Article33. Wardlaw JM, Allerhand M, Doubal FN, Valdes Hernandez M, Morris Z, Gow AJ, et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014; 82:1331–1338. PMID: 24623838.
Article34. Rutten-Jacobs LC, Markus HS. UK Young Lacunar Stroke DNA Study. Vascular risk factor profiles differ between magnetic resonance imaging-defined subtypes of younger-onset lacunar stroke. Stroke. 2017; 48:2405–2411. PMID: 28765289.
Article35. Qiao J, Lu WH, Wang J, Guo XJ, Qu QM. Vascular risk factors aggravate the progression of Alzheimer's disease: a 3-year follow-up study of Chinese population. Am J Alzheimers Dis Other Demen. 2014; 29:521–525. PMID: 24562899.36. Xu X, Hilal S, Collinson SL, Chong EJ, Ikram MK, Venketasubramanian N, et al. Association of magnetic resonance imaging markers of cerebrovascular disease burden and cognition. Stroke. 2015; 46:2808–2814. PMID: 26330446.
Article37. Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology. 2011; 76:1872–1878. PMID: 21543730.
Article38. McAleese KE, Walker L, Graham S, Moya EL, Johnson M, Erskine D, et al. Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017; 134:459–473. PMID: 28638989.
Article39. Grimmer T, Faust M, Auer F, Alexopoulos P, Förstl H, Henriksen G, et al. White matter hyperintensities predict amyloid increase in Alzheimer’s disease. Neurobiol Aging. 2012; 33:2766–2773. PMID: 22410648.
Article40. McAleese KE, Firbank M, Dey M, Colloby SJ, Walker L, Johnson M, et al. Cortical tau load is associated with white matter hyperintensities. Acta Neuropathol Commun. 2015; 3:60. PMID: 26419828.
Article41. Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, et al. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 2014; 71:855–862. PMID: 24818585.
Article42. Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006; 66:23–29. PMID: 16401840.43. Pelletier A, Periot O, Dilharreguy B, Hiba B, Bordessoules M, Chanraud S, et al. Age-related modifications of diffusion tensor imaging parameters and white matter hyperintensities as inter-dependent processes. Front Aging Neurosci. 2016; 7:255. PMID: 26834625.
Article44. Kim S, Choi SH, Lee YM, Kim MJ, Kim YD, Kim JY, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatr. 2015; 27:2069–2077. PMID: 26212042.
Article45. Chen Y, Wang J, Zhang J, Zhang T, Chen K, Fleisher A, et al. Aberrant functional networks connectivity and structural atrophy in silent lacunar infarcts: relationship with cognitive impairments. J Alzheimers Dis. 2014; 42:841–850. PMID: 24946874.
Article46. Nho K, Saykin AJ, Nelson PT. Alzheimer's Disease Neuroimaging Initiative. Hippocampal sclerosis of aging, a common Alzheimer's disease ‘mimic’: risk genotypes are associated with brain atrophy outside the temporal lobe. J Alzheimers Dis. 2016; 52:373–383. PMID: 27003218.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of White-Matter Lesions and Lacunar Infarcts with Cardiovascular Risk Factors
- Effects of Lacunar Infarctions on Cognitive Impairment in Patients with Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
- Is the Severity of Dilated Virchow-Robin Spaces Associated with Cognitive Dysfunction?
- The Correlation of the White Matter Lesions and Lacunar Infarcts in Patients with Vascular Cognitive Impairment
- Prevention and Management of Cerebral Small Vessel Disease