Dement Neurocogn Disord.
2012 Jun;11(2):67-73. 10.12779/dnd.2012.11.2.67.
The Correlation of the White Matter Lesions and Lacunar Infarcts in Patients with Vascular Cognitive Impairment
- Affiliations
-
- 1Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jhlee@amc.seoul.kr
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2172238
- DOI: http://doi.org/10.12779/dnd.2012.11.2.67
Abstract
- BACKGROUND
Cerebral white matter lesions (WMLs) and lacunar infarcts (LIs) are mostly caused by small vessel disease (SVD). Whereas the main pathomechanism behind LIs is SVD, a variety of mechanisms could be responsible for WMLs. We tried to investigate the relationship between WMLs and LIs and the impact of subtypes of WMLs on its relationship.
METHODS
We assessed 128 subjects with vascular cognitive impairment with subcortical vascular lesion (VCI-S). LI number and WML volume were determined on T1-, T2-weighted images and fluid-attenuated inversion recovery images using a semiquantitative visual scale. Cognitive function and daily functional impairment were assessed with Mini-Mental State Examination (MMSE) and the Seoul-Instrumental Activities of Daily Living (S-IADL).
RESULTS
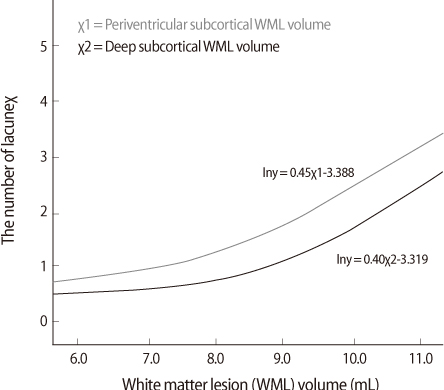
Of the 128 patients, 106 (82.8%) had Alzheimer's disease with WML and 22 (17.2%) had subcortical vascular dementia. Seventy patients (54.7%) had at least one lacune. A univariate Poisson model showed that history of hypertension, history of stroke and WML volume (periventricular and deep subcortical) were associated with LIs. A multivariate Poisson model showed that increased WML volume of both types and history of hypertension were associated with LIs. Neither S-IADL score nor MMSE was significantly associated with WML volume of both types.
CONCLUSIONS
We found that LIs were associated with WMLs regardless of their types in patients with VCI-S. These findings may suggest that periventricular and deep subcortical WMLs share the same vascular pathomechanism of SVD as LIs.
MeSH Terms
Figure
Reference
-
1. Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994. 44:1246–1252.
Article2. Wolma J, Nederkoorn PJ, Goossens A, Vergouwen MD, van Schaik IN, Vermeulen M. Ethnicity a risk factor? The relation between ethnicity and large- and small-vessel disease in White people, Black people, and Asians within a hospital-based population. Eur J Neurol. 2009. 16:522–527.
Article3. Syed NA, Khealani BA, Ali S, Hasan A, Akhtar N, Brohi H, et al. Ischemic stroke subtypes in Pakistan: the Aga Khan University Stroke Data Bank. J Pak Med Assoc. 2003. 53:584–588.4. Deleu D, Hamad AA, Kamram S, El Siddig A, Al Hail H, Hamdy SM. Ethnic variations in risk factor profile, pattern and recurrence of non-cardioembolic ischemic stroke. Arch Med Res. 2006. 37:655–662.
Article5. Kim JS, Yoon SS. Stroke subtypes and risk factors in patients living in southern Seoul, Korea: the impact of hypertension control on stroke subtypes. J Stroke Cerebrovasc Dis. 1998. 7:205–210.
Article6. Lee BI, Nam HS, Heo JH, Kim DI, Team YS. Yonsei Stroke Registry - Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001. 12:145–151.7. Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997. 28:652–659.8. Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, et al. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003. 106:527–534.
Article9. Farkas E, Donka G, de Vos RAI, Mihaly A, Bari F, Luiten PGM. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004. 108:57–64.
Article10. Qiao M, Malisza KL, Del Bigio MR, Tuor UI. Correlation of cerebral hypoxic-ischemic T2 changes with tissue alterations in water content and protein extravasation. Stroke. 2001. 32:958–963.
Article11. Moody DM, Bell MA, Challa VR. Features of the Cerebral Vascular Pattern That Predict Vulnerability to Perfusion or Oxygenation Deficiency - an Anatomic Study. Am J Neuroradiol. 1990. 11:431–439.12. Burns JM, Church JA, Johnson DK, Xiong CJ, Marcus D, Fotenos AF, et al. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol-Chicago. 2005. 62:1870–1876.
Article13. Delano-Wood L, Abeles N, Sacco JM, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment - Differential influence of lesion type on neuropsychological functioning. Stroke. 2008. 39:794–799.
Article14. Soderlund H, Nilsson LG, Berger K, Breteler MM, Dufouil C, Fuhrer R, et al. Cerebral changes on MRI and cognitive function: The CASCADE study. Neurobiol Aging. 2006. 27:16–23.
Article15. Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005. 36:1184–1188.
Article16. Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001. 57:2229–2235.
Article17. de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000. 47:145–151.
Article18. de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002. 52:335–341.
Article19. de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000. 57:1071–1076.
Article20. Stenset V, Hofoss D, Berstad AE, Negaard A, Gjerstad L, Fladby T. White Matter Lesion Subtypes and Cognitive Deficits in Patients with Memory Impairment. Dement Geriatr Cogn Disord. 2008. 26:424–431.
Article21. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. 43:2412–2414.22. Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF. The National Institute of Mental Health Genetics Initiative. Reliability and validity of NINCDS-ADRDA criteria for Alzheimer's disease. Arch Neurol. 1994. 51:1198–1204.
Article23. Roman GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, Lopez-Pousa S, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004. 226:81–87.
Article24. Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008. 7:246–255.
Article25. Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, et al. Impact of age-related cerebral white matter changes on the transition to disability - the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005. 24:51–62.
Article26. Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993. 114:7–12.
Article27. Ku HM, Kim JH, Kwon EJ, Kim SH, Lee HS, Ko HJ, et al. A Study on the Reliability and Validity of Seoul-Instrumental Activities of Daily Living (S-IADL). J Korean Neuropsychiatr Assoc. 2004. 43:189–199.28. Ward NS, Brown MM. Donann G, Narrving B, Bamford J, Bogousslasky J, editors. Leukoaraiosis. Subcortical Stroke. 2002. 2nd ed. Oxford: Oxford University Press;47–66.29. DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005. 36:50–55.
Article30. van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991. 114:761–774.
Article31. Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993. 43:1683–1689.
Article32. van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005. 36:2116–2120.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of White-Matter Lesions and Lacunar Infarcts with Cardiovascular Risk Factors
- Is the Severity of Dilated Virchow-Robin Spaces Associated with Cognitive Dysfunction?
- Effects of Lacunar Infarctions on Cognitive Impairment in Patients with Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy
- Cerebral Small Vessel Disease and Chronic Kidney Disease
- Can We Further Divide Amnestic Mild Cognitive Impairment Based on the Pattern of Memory Deficit?: A Preliminary Study