Dement Neurocogn Disord.
2015 Sep;14(3):114-119. 10.12779/dnd.2015.14.3.114.
Is the Severity of Dilated Virchow-Robin Spaces Associated with Cognitive Dysfunction?
- Affiliations
-
- 1Department of Neurology, Bundang Jesaeng General Hospital, Seongnam, Korea.
- 2Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jhlee@amc.seoul.kr
- 3Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2442986
- DOI: http://doi.org/10.12779/dnd.2015.14.3.114
Abstract
- BACKGROUND AND PURPOSE
Dilated Virchow-Robin spaces (dVRS) are not uncommon findings in the normal brain, particularly in the old people, and have been largely regarded as benign lesions. However, there is accumulating evidence that dVRS may serve as an neuroimaging marker of small vessel disease and are associated with cognitive decline. We investigated whether the severity of dVRS would be associated with cognitive dysfunction by comparing the subjects with subjective memory impairment (SMI), mild cognitive impairment (MCI), and Alzheimer's disease (AD). We also examined whether there were differences in the degree of correlation between dVRS and magnetic resonance imaging (MRI) markers of small vessel disease among the three groups.
METHODS
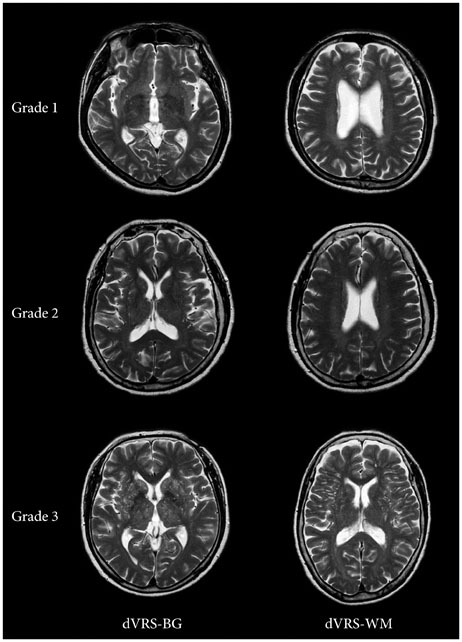
In this retrospective study, a total of 225 subjects were included: those with SMI (n=65), MCI (n=100), and AD (n=60). We rated the severity of dVRS using the axial MRI slice containing the greatest number of dVRS in the basal ganglia (dVRS-BG) and in the deep white matter (dVRS-WM), separately. We also assessed baseline characteristics including vascular risk factors and MRI markers of small vessel disease such as white matter hyperintensities (WMH), lacunar infarcts and microbleeds.
RESULTS
A cumulative logit model revealed that the severity of cognitive dysfunction was associated with age (p<0.001), hypertension (p=0.006), diabetes mellitus (p=0.042), the severity of dVRS-BG (p=0.001), the severity of WMH (p=0.074) and the presence of lacunar infarcts (p<0.001) and microbleeds (p=0.003) in univariate analysis. However, after adjusting for other confounding variables, the severity of dVRS-BG was not a significant discriminating factor among subjects with SMI, MCI, and AD. Spearman's correlation analysis showed a trend that the correlation between the severity of dVRS-BG and the severity of WMH became more prominent in subjects with AD than in those with MCI or SMI (r=0.191 in SMI; r=0.284 in MCI; r=0.312 in AD), and the same is true of the severity of dVRS-BG and the number of lacunar infarcts.
CONCLUSIONS
The severity of dVRS was associated with cognitive dysfunction, which appeared to be confounded by other well-known risk factors. The correlation between dVRS-BG and small vessel disease markers tended to be more significant with the advancement of cognitive impairment. These results suggest that severe dVRS may reflect cerebral small vessel disease and contribute to cognitive impairment.
Keyword
MeSH Terms
Figure
Reference
-
1. Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007; 27:1071–1086.2. Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008; 255:692–696.
Article3. Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006; 238:962–974.
Article4. Zhu YC, Dufouil C, Mazoyer B, Soumaré A, Ricolfi F, Tzourio C, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol. 2011; 32:709–713.5. Jungreis CA, Kanal E, Hirsch WL, Martinez AJ, Moossy J. Normal perivascular spaces mimicking lacunar infarction: MR imaging. Radiology. 1988; 169:101–104.6. Benhaïem-Sigaux N, Gray F, Gherardi R, Roucayrol AM, Poirier J. Expanding cerebellar lacunes due to dilatation of the perivascular space associated with Binswanger's subcortical arteriosclerotic encephalopathy. Stroke. 1987; 18:1087–1092.
Article7. Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997; 191(Pt 3):337–346.
Article8. Homeyer P, Cornu P, Lacomblez L, Chiras J, Derouesné C. A special form of cerebral lacunae: expanding lacunae. J Neurol Neurosurg Psychiatry. 1996; 61:200–202.
Article9. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010; 41:450–454.
Article10. Zhu YC, Dufouil C, Soumaré A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimers Dis. 2010; 22:663–672.
Article11. Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, et al. Perivascular spaces--MRI marker of inflammatory activity in the brain? Brain. 2008; 131(Pt 9):2332–2340.
Article12. Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004; 75:1519–1523.
Article13. Weller RO, Yow HY, Preston SD, Mazanti I, Nicoll JA. Cerebrovascular disease is a major factor in the failure of elimination of Abeta from the aging human brain: implications for therapy of Alzheimer's disease. Ann N Y Acad Sci. 2002; 977:162–168.
Article14. Kang YW, Na DL. Seoul neuropsychological screening battery. Incheon: Human Brain Research & Consulting Co.;2003.
Article15. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004; 256:183–194.
Article16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association;2000.17. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.18. Moon SY, Na DL, Seo SW, Lee JY, Ku BD, Kim SY, et al. Impact of white matter changes on activities of daily living in mild to moderate dementia. Eur Neurol. 2011; 65:223–230.
Article19. Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, et al. Cortical thickness in single-versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007; 36:289–297.
Article20. Chen W, Song X, Zhang Y. Alzheimer's Disease Neuroimaging Initiative. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. AJNR Am J Neuroradiol. 2011; 32:1490–1495.
Article21. Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis. 2015; 43:415–424.
Article22. Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005; 26:1512–1520.
Article23. Hansen TP, Cain J, Thomas O, Jackson A. Dilated perivascular spaces in the Basal Ganglia are a biomarker of small-vessel disease in a very elderly population with dementia. AJNR Am J Neuroradiol. 2015; 36:893–898.
Article24. Gold G, Kövari E, Herrmann FR, Canuto A, Hof PR, Michel JP. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005; 36:1184–1188.
Article25. Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol. 2009; 118:87–102.
Article26. van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991; 114(Pt 2):761–774.
Article27. Adams HH, Cavalieri M, Verhaaren BF, Bos D, van der Lugt A, Enzinger C, et al. Rating method for dilated Virchow-Robin spaces on magnetic resonance imaging. Stroke. 2013; 44:1732–1735.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MRI findings of cryptococcal infection of CNS: The long term follow-up: case report
- A Case of Meningioma in Temporo-occipital Lobe without Dural Attachment in a 14-yer-old Girl: Case Report
- A Case of Huge Reticulum Cell Sarcoma of the Brain
- Cognitive Dysfunction and Diabetes
- A Case of Pierre Robin Syndrome