J Clin Neurol.
2019 Jul;15(3):301-307. 10.3988/jcn.2019.15.3.301.
Impaired Smooth Pursuit During Transient Global Amnesia
- Affiliations
-
- 1Department of Neurology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea.
- 2Department of Neurology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seoul, Korea. jisookim@snu.ac.kr
- KMID: 2451112
- DOI: http://doi.org/10.3988/jcn.2019.15.3.301
Abstract
- BACKGROUND AND PURPOSE
During transient global amnesia (TGA), selective impairment of episodic memory is assumed to occur due to alteration in the neuronal network between the hippocampus and parietooccipital cortices that also include a hub for smooth pursuit (SP) eye movements. This study aimed to determine whether SP is impaired during TGA, and to identify any anatomical and functional linkage present between the oculomotor and memory systems.
METHODS
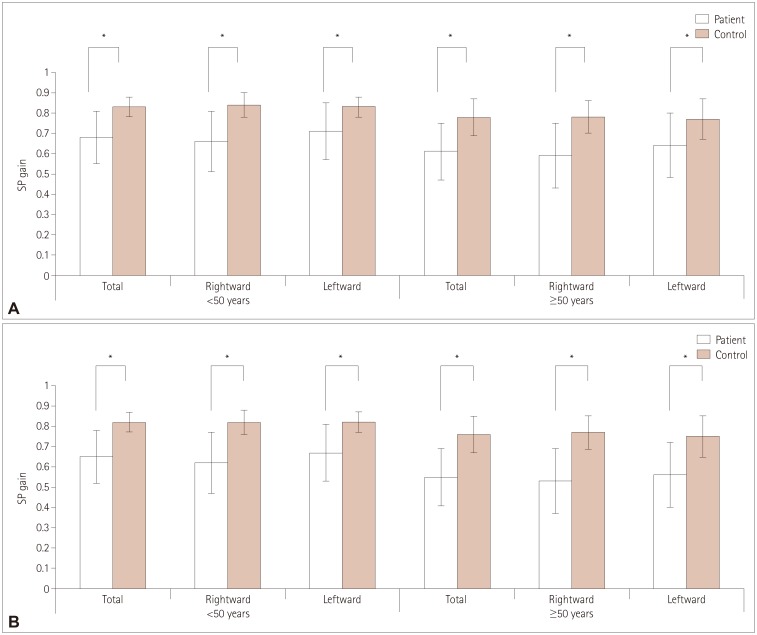
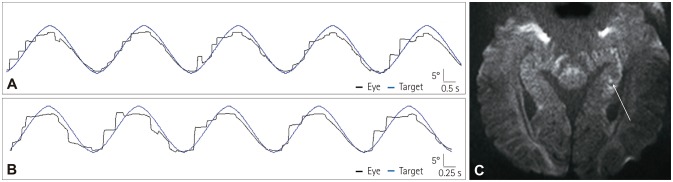
Within a median of 1.0 day of TGA, horizontal SP was evaluated in 145 patients with a target moving at peak velocities of 10°/s and 20°/s. The average SP gains of patients were compared with those of the age-matched controls.
RESULTS
The patients with TGA showed lower SP gains in both directions for both peak target velocities. While the normal controls showed symmetric SP in the rightward and leftward directions, in the TGA patients the SP gain was lower during rightward than leftward SP regardless of bilaterality or the side of the lesions.
CONCLUSIONS
The cortical regions processing information about visual motion appeared to be affected during or soon after an amnestic episode of TGA, and more so in the right hemisphere. This means that disturbed processing of dynamic visual information may be related to the impaired spatial orientation observed during TGA.
MeSH Terms
Figure
Reference
-
1. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990; 53:834–843. PMID: 2266362.
Article2. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010; 9:205–214. PMID: 20129169.
Article3. Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000; 911:25–42. PMID: 10911865.
Article4. Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci. 2012; 32:6550–6560. PMID: 22573677.
Article5. Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012; 13:713–726. PMID: 22992647.
Article6. La Joie R, Landeau B, Perrotin A, Bejanin A, Egret S, Pélerin A, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron. 2014; 81:1417–1428. PMID: 24656258.
Article7. Yi S, Park YH, Jang JW, Lim JS, Chun IK, Kim S. Decreased metabolism in the posterior medial network with concomitantly increased metabolism in the anterior temporal network during transient global amnesia. Brain Topogr. 2018; 31:468–476. PMID: 29038979.
Article8. Park YH, Jeong HY, Jang JW, Park SY, Lim JS, Kim JY, et al. Disruption of the posterior medial network during the acute stage of transient global amnesia: a preliminary study. Clin EEG Neurosci. 2016; 47:69–74. PMID: 25392008.9. Trenner MU, Fahle M, Fasold O, Heekeren HR, Villringer A, Wenzel R. Human cortical areas involved in sustaining perceptual stability during smooth pursuit eye movements. Hum Brain Mapp. 2008; 29:300–311. PMID: 17415782.
Article10. Morrow MJ, Sharpe JA. Cerebral hemispheric localization of smooth pursuit asymmetry. Neurology. 1990; 40:284–292. PMID: 2300251.
Article11. Lencer R, Trillenberg P. Neurophysiology and neuroanatomy of smooth pursuit in humans. Brain Cogn. 2008; 68:219–228. PMID: 18835076.
Article12. Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989; 62:31–47. PMID: 2754480.
Article13. Mikami A, Newsome WT, Wurtz RH. Motion selectivity in macaque visual cortex I Mechanisms of direction and speed selectivity in extrastriate area MT. J Neurophysiol. 1986; 55:1308–1327. PMID: 3016210.
Article14. Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988; 60:940–965. PMID: 3171667.15. Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004; 8:315–324. PMID: 15242691.
Article16. Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 2008; 68:309–326. PMID: 18848744.
Article17. Kerzel D, Souto D, Ziegler NE. Effects of attention shifts to stationary objects during steady-state smooth pursuit eye movements. Vision Res. 2008; 48:958–969. PMID: 18295816.
Article18. Treue S, Maunsell JH. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci. 1999; 19:7591–7602. PMID: 10460265.
Article19. Contreras R, Ghajar J, Bahar S, Suh M. Effect of cognitive load on eyetarget synchronization during smooth pursuit eye movement. Brain Res. 2011; 1398:55–63. PMID: 21620377.
Article20. Hutton SB, Tegally D. The effects of dividing attention on smooth pursuit eye tracking. Exp Brain Res. 2005; 163:306–313. PMID: 15654587.
Article21. Chung YA, Jeong J, Yang DW, Kang BJ, Kim SH, Chung SK, et al. A Tc-99m SPECT study of regional cerebral blood flow in patients with transient global amnesia. Neuroimage. 2009; 47:50–55. PMID: 19073268.
Article22. Kim BS, Cho SS, Choi JY, Kim YH. Transient global amnesia: a study with Tc-99m ECD SPECT shortly after symptom onset and after recovery. Diagn Interv Radiol. 2016; 22:476–480. PMID: 27535207.
Article23. Matsuda H, Higashi S, Tsuji S, Sumiya H, Miyauchi T, Hisada K, et al. High resolution Tc-99m HMPAO SPECT in a patient with transient global amnesia. Clin Nucl Med. 1993; 18:46–49. PMID: 8422720.
Article24. Segraves MA, Goldberg ME, Deng SY, Bruce CJ, Ungerleider LG, Mishkin M. The role of striate cortex in the guidance of eye movements in the monkey. J Neurosci. 1987; 7:3040–3058. PMID: 3668615.
Article25. Robinson DA. The mechanics of human smooth pursuit eye movement. J Physiol. 1965; 180:569–591. PMID: 5846794.
Article26. Ono S, Mustari MJ. Smooth pursuit-related information processing in frontal eye field neurons that project to the NRTP. Cereb Cortex. 2009; 19:1186–1197. PMID: 18820288.
Article27. Lindner A, Haarmeier T, Erb M, Grodd W, Thier P. Cerebrocerebellar circuits for the perceptual cancellation of eye-movement-induced retinal image motion. J Cogn Neurosci. 2006; 18:1899–1912. PMID: 17069480.
Article28. Yang Y, Kim JS, Kim S, Kim YK, Kwak YT, Han IW. Cerebellar hypoperfusion during transient global amnesia: an MRI and oculographic study. J Clin Neurol. 2009; 5:74–80. PMID: 19587813.
Article29. Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res. 1991; 11:79–107. PMID: 1656345.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transient Global Amnesia after Gastroscopy
- Recurrent transient amnesia: a case of transient epileptic amnesia misdiag-nosed as transient global amnesia
- Transient Global Amnesia Developed after Zolpidem Intake
- Cerebellar Hypoperfusion during Transient Global Amnesia: An MRI and Oculographic Study
- Retrograde Amnesia as a Predominant Symptom of Transient Global Amnesia