Ann Lab Med.
2019 Sep;39(5):421-429. 10.3343/alm.2019.39.5.421.
Challenges and Considerations in Sequence Variant Interpretation for Mendelian Disorders
- Affiliations
-
- 1Department of Laboratory Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 2Green Cross Genome, Yongin, Korea.
- 3Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. miaeyaho@schmc.ac.kr
- KMID: 2450964
- DOI: http://doi.org/10.3343/alm.2019.39.5.421
Abstract
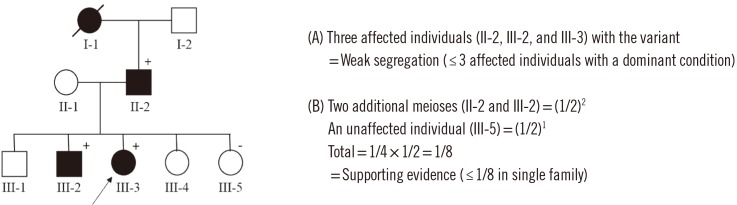
- In 2015, the American College of Medical Genetics and Genomics (ACMG), together with the Association for Molecular Pathology (AMP), published the latest guidelines for the interpretation of sequence variants, which have been widely adopted into clinical practice. Despite these standardized efforts, the degrees of subjectivity and uncertainty allowed by the guidelines can lead to inconsistent variant classification across clinical laboratories, making it difficult to assess the pathogenicity of identified variants. We describe the critical elements of variant interpretation processes and potential pitfalls through practical examples and provide updated information based on a review of recent literature. The variant classification we describe is meant to be applicable to sequence variants for Mendelian disorders, whether identified by single-gene tests, multi-gene panels, exome sequencing, or genome sequencing. Continuing efforts to improve the reproducibility and objectivity of sequence variant interpretation across individuals and laboratories are needed.
MeSH Terms
Figure
Cited by 1 articles
-
Application of Next Generation Sequencing in Laboratory Medicine
Yiming Zhong, Feng Xu, Jinhua Wu, Jeffrey Schubert, Marilyn M. Li
Ann Lab Med. 2021;41(1):25-43. doi: 10.3343/alm.2021.41.1.25.
Reference
-
1. Vears DF, Senecal K, Borry P. Reporting practices for variants of uncertain significance from next generation sequencing technologies. Eur J Med Genet. 2017; 60:553–558. PMID: 28774848.2. Kazazian HH, Boehm CD, Seltzer WK. ACMG recommendation for standards for interpretation of sequence variations. Genet Med. 2000; 2:302–303.3. Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, et al. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008; 10:294–300. PMID: 18414213.4. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.5. Harrison SM, Dolinsky JS, Knight Johnson AE, Pesaran T, Azzariti DR, Bale S, et al. Clinical laboratories collaborate to resolve differences in variant interpretations submitted to ClinVar. Genet Med. 2017; 19:1096–1104. PMID: 28301460.6. Balmaña J, Digiovanni L, Gaddam P, Walsh MF, Joseph V, Stadler ZK, et al. Conflicting interpretation of genetic variants and cancer risk by commercial laboratories as assessed by the Prospective Registry of Multiplex Testing. J Clin Oncol. 2016; 34:4071–4078. PMID: 27621404.7. Amendola LM, Jarvik GP, Leo MC, McLaughlin HM, Akkari Y, Amaral MD, et al. Performance of ACMG-AMP variant-interpretation guidelines among nine laboratories in the Clinical Sequencing Exploratory Research Consortium. Am J Hum Genet. 2016; 98:1067–1076. PMID: 27181684.8. Pepin MG, Murray ML, Bailey S, Leistritz-Kessler D, Schwarze U, Byers PH. The challenge of comprehensive and consistent sequence variant interpretation between clinical laboratories. Genet Med. 2016; 18:20–24. PMID: 25834947.9. Hoskinson DC, Dubuc AM, Mason-Suares H. The current state of clinical interpretation of sequence variants. Curr Opin Genet Dev. 2017; 42:33–39. PMID: 28157586.10. Nykamp K, Anderson M, Powers M, Garcia J, Herrera B, Ho YY, et al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 2017; 19:1105–1117. PMID: 28492532.11. 1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015; 526:68–74. PMID: 26432245.12. Tennessen JA, Bigham AW, O'Connor TD, Fu W, Kenny EE, Gravel S, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012; 337:64–69. PMID: 22604720.13. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016; 536:285–291. PMID: 27535533.14. 1000 Genomes Project Consortium. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010; 467:1061–1073. PMID: 20981092.15. Norton N, Robertson PD, Rieder MJ, Züchner S, Rampersaud E, Martin E, et al. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ Cardiovasc Genet. 2012; 5:167–174. PMID: 22337857.16. Shearer AE, Eppsteiner RW, Booth KT, Ephraim SS, Gurrola J 2nd, Simpson A, et al. Utilizing ethnic-specific differences in minor allele frequency to recategorize reported pathogenic deafness variants. Am J Hum Genet. 2014; 95:445–453. PMID: 25262649.17. Kobayashi Y, Yang S, Nykamp K, Garcia J, Lincoln SE, Topper SE. Pathogenic variant burden in the ExAC database: an empirical approach to evaluating population data for clinical variant interpretation. Genome Med. 2017; 9:13. PMID: 28166811.18. Duzkale H, Shen J, McLaughlin H, Alfares A, Kelly MA, Pugh TJ, et al. A systematic approach to assessing the clinical significance of genetic variants. Clin Genet. 2013; 84:453–463. PMID: 24033266.19. Whiffin N, Minikel E, Walsh R, O'Donnell-Luria AH, Karczewski K, Ing AY, et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med. 2017; 19:1151–1158. PMID: 28518168.20. Kelly MA, Caleshu C, Morales A, Buchan J, Wolf Z, Harrison SM, et al. Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: recommendations by ClinGen's Inherited Cardiomyopathy Expert Panel. Genet Med. 2018; 20:351–359. PMID: 29300372.21. Jang MA, Shin S, Yoon JH, Ki CS. Frequency of the moyamoya-related RNF213 p.Arg4810Lys variant in 1,516 Korean individuals. BMC Med Genet. 2015; 16:109. PMID: 26590131.22. Tabor HK, Auer PL, Jamal SM, Chong JX, Yu JH, Gordon AS, et al. Pathogenic variants for Mendelian and complex traits in exomes of 6,517 European and African Americans: implications for the return of incidental results. Am J Hum Genet. 2014; 95:183–193. PMID: 25087612.23. Park JS, Nam EJ, Park HS, Han JW, Lee JY, Kim J, et al. Identification of a novel BRCA1 pathogenic mutation in Korean patients following reclassification of BRCA1 and BRCA2 variants according to the ACMG standards and guidelines using relevant ethnic controls. Cancer Res Treat. 2017; 49:1012–1021. PMID: 28111427.24. Kim YE, Yoon CW, Seo SW, Ki CS, Kim YB, Kim JW, et al. Spectrum of NOTCH3 mutations in Korean patients with clinically suspicious cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging. 2014; 35:726.e1–726.e6.25. Muiño E, Gallego-Fabrega C, Cullell N, Carrera C, Torres N, Krupinski J, et al. Systematic review of cysteine-sparing NOTCH3 missense mutations in patients with clinical suspicion of CADASIL. Int J Mol Sci. 2017; 18:E1964. PMID: 28902129.26. Ghosh R, Oak N, Plon SE. Evaluation of in silico algorithms for use with ACMG/AMP clinical variant interpretation guidelines. Genome Biol. 2017; 18:225. PMID: 29179779.27. Lee J, Park J, Choi H, Kim J, Kwon A, Jang W, et al. Genetic profiles of Korean patients with glucose-6-phosphate dehydrogenase deficiency. Ann Lab Med. 2017; 37:108–116. PMID: 28028996.28. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010; 7:248–249. PMID: 20354512.29. Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003; 31:3812–3814. PMID: 12824425.30. Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012; 7:e46688. PMID: 23056405.31. Thusberg J, Olatubosun A, Vihinen M. Performance of mutation pathogenicity prediction methods on missense variants. Hum Mutat. 2011; 32:358–368. PMID: 21412949.32. Tang H, Thomas PD. Tools for predicting the functional impact of nonsynonymous genetic variation. Genetics. 2016; 203:635–647. PMID: 27270698.33. Grimm DG, Azencott CA, Aicheler F, Gieraths U, MacArthur DG, Samocha KE, et al. The evaluation of tools used to predict the impact of missense variants is hindered by two types of circularity. Hum Mutat. 2015; 36:513–523. PMID: 25684150.34. Maxwell KN, Hart SN, Vijai J, Schrader KA, Slavin TP, Thomas T, et al. Evaluation of ACMG-guideline-based variant classification of cancer susceptibility and non-cancer-associated genes in families affected by breast cancer. Am J Hum Genet. 2016; 98:801–817. PMID: 27153395.35. Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013; 45:1160–1167. PMID: 23974870.36. Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014; 46:310–315. PMID: 24487276.37. Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat. 2011; 32:894–899. PMID: 21520341.38. Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat Genet. 2016; 48:1581–1586. PMID: 27776117.39. Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying Mendelian disease genes with the variant effect scoring tool. BMC Genomics. 2013; 14(Suppl 3):S3.40. Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016; 99:877–885. PMID: 27666373.41. Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet. 2015; 24:2125–2137. PMID: 25552646.42. Choi JC. Cerebral autosomal dominant arteriopathy with subcortical in farcts and leukoencephalopathy: a genetic cause of cerebral small vessel disease. J Clin Neurol. 2010; 6:1–9. PMID: 20386637.43. Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009; 8:643–653. PMID: 19539236.44. Jarvik GP, Browning BL. Consideration of cosegregation in the pathogenicity classification of genomic variants. Am J Hum Genet. 2016; 98:1077–1081. PMID: 27236918.45. MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014; 508:469–476. PMID: 24759409.46. Gelb BD, Cavé H, Dillon MW, Gripp KW, Lee JA, Mason-Suares H, et al. ClinGen's RASopathy Expert Panel consensus methods for variant interpretation. Genet Med. 2018; 20:1334–1345. PMID: 29493581.47. Strande NT, Brnich SE, Roman TS, Berg JS. Navigating the nuances of clinical sequence variant interpretation in Mendelian disease. Genet Med. 2018; 20:918–926. PMID: 29988079.48. Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, et al. ClinGen-the clinical genome resource. N Engl J Med. 2015; 372:2235–2242. PMID: 26014595.49. Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014; 42:D980–D985. PMID: 24234437.50. Li Q, Wang K. InterVar: Clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am J Hum Genet. 2017; 100:267–280. PMID: 28132688.51. Li Z, Liu Z, Jiang Y, Chen D, Ran X, Sun ZS, et al. mirVAFC: A web server for prioritizations of pathogenic sequence variants from exome sequencing data via classifications. Hum Mutat. 2017; 38:25–33. PMID: 27676360.52. The human genomics variant search engine (VarSome). Updated on Aug 2018. https://varsome.com.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Challenges and Considerations in Sequence Variant Interpretation for Mendelian Disorders
- Exome Sequencing in Mendelian Disorders
- Clinical applications of next-generation sequencing in the diagnosis of genetic disorders in Korea: a narrative review
- Applying Functional Assay Evidence to Interpret Sequence Variants Identified in Hereditary Cancer Genes
- Basic Concepts of a Mendelian Randomization Approach