Ann Lab Med.
2019 Nov;39(6):590-592. 10.3343/alm.2019.39.6.590.
Accuracy of BacT/Alert Virtuo for Measuring Blood Volume for Blood Culture
- Affiliations
-
- 1Department of Laboratory Medicine, Geyongsang National University Changwon Hospital, Changwon, Korea. sjkim8239@hanmail.net
- 2Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- KMID: 2450958
- DOI: http://doi.org/10.3343/alm.2019.39.6.590
Abstract
- No abstract available.
MeSH Terms
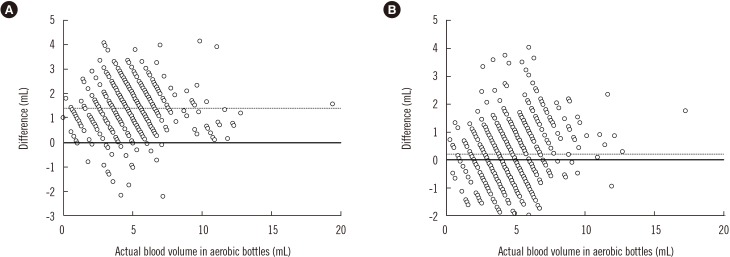
Figure
Cited by 1 articles
-
Effect of Wearing Personal Protective Equipment (PPE) for COVID-19 Treatment on Blood Culture Contamination: Implication for Optimal PPE Strategies
Jae Hyeon Park, Taek Soo Kim, Chan Mi Lee, Chang Kyung Kang, Wan Beom Park, Nam Joong Kim, Pyoeng Gyun Choe, Myoung-don Oh
J Korean Med Sci. 2023;38(23):e180. doi: 10.3346/jkms.2023.38.e180.
Reference
-
1. Lamy B, Ferroni A, Henning C, Cattoen C, Laudat P. How to: accreditation of blood cultures' proceedings. A clinical microbiology approach for adding value to patient care. Clin Microbiol Infect. 2018; 24:956–963. PMID: 29410246.2. CLSI. Principles and procedures for blood cultures; approved guideline. M47-A. Wayne, PA: Clinical and Laboratory Standards Institute;2007.3. Coorevits L, Van den Abeele AM. Evaluation of the BD BACTEC FX blood volume monitoring system as a continuous quality improvement measure. Eur J Clin Microbiol Infect Dis. 2015; 34:1459–1466. PMID: 25894984.4. Cattoir L, Claessens J, Cartuyvels R, Van den Abeele AM. How to achieve accurate blood culture volumes: the BD BACTEC FX blood volume monitoring system as a measuring instrument and educational tool. Eur J Clin Microbiol Infect Dis. 2018; 37:1621–1626. PMID: 29882176.5. Trudnowski RJ, Rico RC. Specific gravity of blood and plasma at 4 and 37 degrees C. Clin Chem. 1974; 20:615–616. PMID: 4826961.6. Jones RL, Sayles HR, Fey PD, Rupp ME. Effect of clinical variables on the volume of blood collected for blood cultures in an adult patient population. Infect Control Hosp Epidemiol. 2017; 38:1493–1497. PMID: 29157318.7. Chang J, Park JS, Park S, Choi B, Yoon NS, Sung H, et al. Impact of monitoring blood volume in the BD BACTEC FX blood culture system: virtual volume versus actual volume. Diagn Microbiol Infect Dis. 2015; 81:89–93. PMID: 25433403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Clinical Performance Between BacT/Alert Virtuo and BacT/Alert 3D Blood Culture Systems
- Comparison of the BACTEC Peds Plus Pediatric Blood Culture Bottle to the BacT/Alert PF Pediatric Blood Culture Bottle for Culturing Blood from Pediatric Patients
- Evaluation of Positive Rate of Aerobic BacT/Alert Blood Culture Bottles by Antibiotic Usage and Inoculated Blood Volume
- Effect of Preincubation of Blood Culture Bottles in a BacT/Alert Unit Outside Laboratory Operating Hours on Detection Time
- Comparison of BACTEC Plus Aerobic/F Media and BacT/Alert FA Media to Detect Bacteria in Blood Culture Bottles Containing Peak Therapeutic Levels of Antimicrobials