Korean J Clin Microbiol.
2011 Dec;14(4):126-130. 10.5145/KJCM.2011.14.4.126.
Comparison of the BACTEC Peds Plus Pediatric Blood Culture Bottle to the BacT/Alert PF Pediatric Blood Culture Bottle for Culturing Blood from Pediatric Patients
- Affiliations
-
- 1Department of Pediatrics, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea. swonkeun@hallym.or.kr
- KMID: 1476033
- DOI: http://doi.org/10.5145/KJCM.2011.14.4.126
Abstract
- BACKGROUND
We compared the BACTEC Peds Plus (Becton Dickinson, USA) and BacT/Alert PF (bioMerieux, France) pediatric blood culture bottles in the context of recovery and time to detection (TTD) of bacteria and fungi from pediatric patients.
METHODS
Blood samples were collected for culture from pediatric patients who were hospitalized during 2010 at a university hospital. BACTEC Peds Plus and BacT/Alert PF bottles were placed in the BACTEC FX and BacT/Alert 3D blood culture system, respectively, and tested for 5 days. Bottles flagged by instruments as positive were removed from the instruments and the TTDs were recorded.
RESULTS
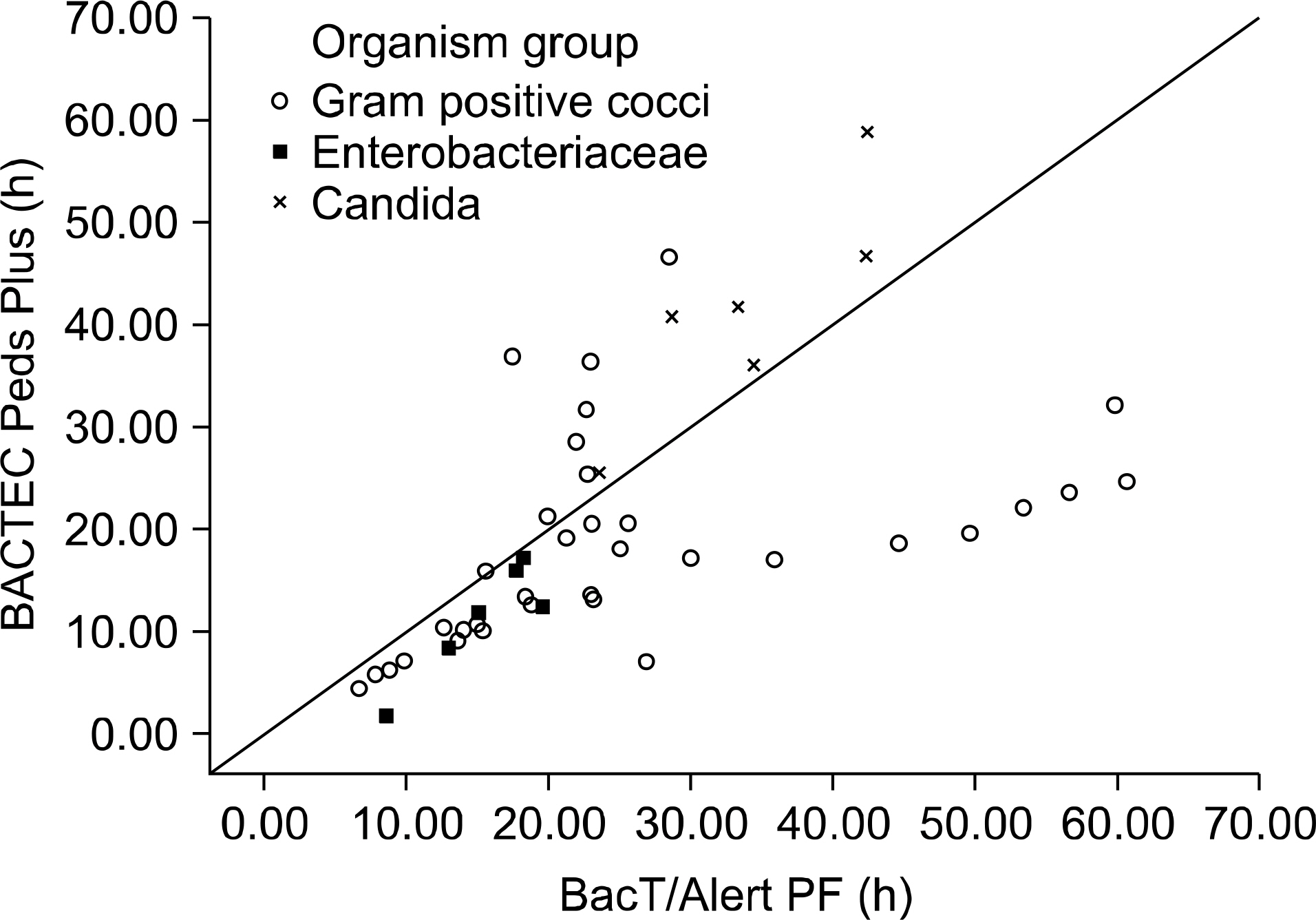
A total of 5,018 sets (1 set, 1 BACTEC Peds Plus and 1 BacT/Alert PF) were evaluated. Overall, the recovery proportions for BACTEC Peds Plus and BacT/Alert PF bottles were 57% (134/195) and 69% (112/195), respectively. There was a significant difference between the 0.38% contamination rate in BacT/Alert PF bottles and the 0.16% contamination rate in BACTEC Peds Plus bottles (P=0.035). The average TTD for all microorganisms was significantly decreased for the BACTEC Peds Plus bottles (P=0.021), but was increased for Candida parapsilosis compared to the results for the BacT/Alert PF bottles (P=0.028).
CONCLUSION
We conclude that the rate of detection and contamination is higher when BacT/Alert PF bottles are used than when BACTEC Peds Plus bottles are used for pediatric blood culture. The BACTEC Peds Plus bottles detect nearly all enrolled microorganisms significantly faster than do the BacT/Alert PF bottles.
Figure
Reference
-
1. Shafazand S and Weinacker AB. Blood cultures in the critical care unit: improving utilization and yield. Chest. 2002; 122:1727–36.2. Ziegler R, Johnscher I, Martus P, Lenhardt D, Just HM. Controlled clinical laboratory comparison of two supplemented aerobic and anaerobic media used in automated blood culture systems to detect bloodstream infections. J Clin Microbiol. 1998; 36:657–61.
Article3. Spaargaren J, van Boven CP, Voorn GP. Effectiveness of resins in neutralizing antibiotic activities in bactec plus Aerobic/F culture medium. J Clin Microbiol. 1998; 36:3731–3.
Article4. Rohner P, Pepey B, Auckenthaler R. Advantage of combining resin with lytic BACTEC blood culture media. J Clin Microbiol. 1997; 35:2634–8.
Article5. Mirrett S, Everts RJ, Reller LB. Controlled comparison of original vented aerobic fan medium with new nonvented BacT/ALERT FA medium for culturing blood. J Clin Microbiol. 2001; 39:2098–101.
Article6. Wilson ML, Mirrett S, Meredith FT, Weinstein MP, Scotto V, Reller LB. Controlled clinical comparison of BACTEC plus anaerobic/F to standard anaerobic/F as the anaerobic companion bottle to plus aerobic/F medium for culturing blood from adults. J Clin Microbiol. 2001; 39:983–9.
Article7. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997; 24:584–602.
Article8. Park SH, Shim H, Yoon NS, Kim MN. Clinical relevance of time-to-positivity in BACTEC9240 blood culture system. Korean J Lab Med. 2010; 30:276–83.
Article9. Krisher KK, Gibb P, Corbett S, Church D. Comparison of the BacT/Alert PF pediatric FAN blood culture bottle with the standard pediatric blood culture bottle, the Pedi-BacT. J Clin Microbiol. 2001; 39:2880–3.
Article10. Haimi-Cohen Y, Shafinoori S, Tucci V, Rubin LG. Use of incubation time to detection in BACTEC 9240 to distinguish coagulase-negative staphylococcal contamination from infection in pediatric blood cultures. Pediatr Infect Dis J. 2003; 22:968–74.
Article11. Gonsalves WI, Cornish N, Moore M, Chen A, Varman M. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol. 2009; 47:3482–5.
Article12. Jorgensen JH, Mirrett S, McDonald LC, Murray PR, Weinstein MP, Fune J, et al. Controlled clinical laboratory comparison of BACTEC plus aerobic/F resin medium with BacT/Alert aerobic FAN medium for detection of bacteremia and fungemia. J Clin Microbiol. 1997; 35:53–8.
Article13. Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. Comparison of BACTEC PLUS blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J Clin Microbiol. 2007; 45:816–21.
Article14. Lee JY, Hong JH, Lee M. Comparison of BACTEC Plus aerobic/F media and BacT/Alert FA media to detect bacteria in blood culture bottles containing peak therapeutic levels of antimicrobials. Korean J Clin Microbiol. 2010; 13:151–6.
Article15. Viganò EF, Vasconi E, Agrappi C, Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT and BACTEC 9240 blood culture systems. Diagn Microbiol Infect Dis. 2002; 44:235–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Comparison of the BACTEC Peds Plus Pediatric Blood Culture Bottle to the BacT/Alert PF Pediatric Blood Culture Bottle for Culturing Blood from Pediatric Patients
- Evaluation of Positive Rate of Aerobic BacT/Alert Blood Culture Bottles by Antibiotic Usage and Inoculated Blood Volume
- Comparison of BACTEC Plus Aerobic/F Media and BacT/Alert FA Media to Detect Bacteria in Blood Culture Bottles Containing Peak Therapeutic Levels of Antimicrobials
- The Effectiveness of Pediatric Blood Culture Bottle in Endophthalmitis
- Comparison of the BACTEC Blood Culture and Conventional Culture Methods for Isolation of Microorganisms Causing Peritonitis in CAPD Patients