Investig Clin Urol.
2019 Jul;60(4):258-266. 10.4111/icu.2019.60.4.258.
Dynamic thiol/disulfide homeostasis as a novel indicator of oxidative stress in patients with urolithiasis
- Affiliations
-
- 1Department of Urology, Meram Medical Faculty, Necmettin Erbakan University, Konya, Turkey. drgiraysonmez@gmail.com
- 2Department of Anesthesiology and Reanimation, University of Health Sciences, Konya Training and Research Hospital, Konya, Turkey.
- 3Department of Medical Biochemistry, University of Health Sciences, Konya Training and Research Hospital, Konya, Turkey.
- 4Department of Medical Education and Informatics, Meram Medical Faculty, Necmettin Erbakan University, Konya, Turkey.
- 5Department of Biochemistry, Yildirim Beyazit University, School of Medicine, Ankara Ataturk Teaching and Research Hospital, Ankara, Turkey.
- KMID: 2450543
- DOI: http://doi.org/10.4111/icu.2019.60.4.258
Abstract
- PURPOSE
A dynamic thiol/disulfide balance is pivotal in organizing anti-oxidant defense, detoxification, apoptosis, and enzyme activities, as well as transcription and cellular signal-transfer mechanisms. The connection between urolithiasis and oxidant/antioxidant status, which can be assessed through thiol-disulfide homeostasis (TDH), has not yet been examined. In this study, we evaluated the effects of TDH on the formation, size, and location of stones by examining the associations between TDH parameters and urolithiasis.
MATERIALS AND METHODS
Patients with urolithiasis and healthy controls were recruited. The patients were divided into subgroups in terms of stone size (>15 mm or ≤15 mm) and stone location (nephrolithiasis or ureterolithiasis). TDH parameters were measured using a novel automatic and spectrophotometric method and compared statistically.
RESULTS
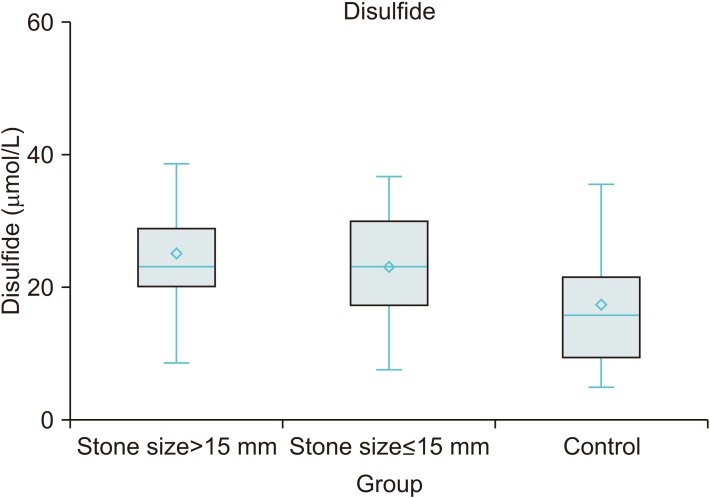
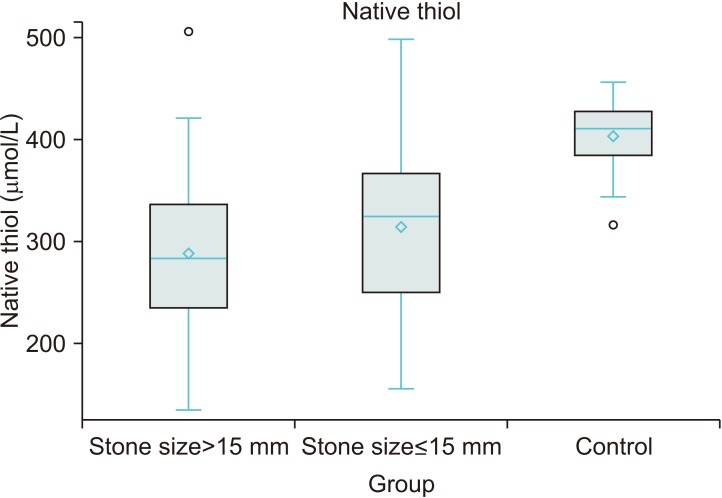
TDH parameters were different between the urolithiasis and control groups. TDH tended towards the disulfide side in the urolithiasis group. Stone size increased an average 0.14 mm with a 1 µmol/L increase in disulfide level and decreased an average 0.058 mm with a 1 µmol/L increase in native thiol level. Disulfide and native thiol levels were found to be different across patients with stone size >15 mm, ≤15 mm, and controls (p<0.001 and p<0.001, respectively). However, the nephrolithiasis and ureterolithiasis groups were similar in respect of TDH parameters.
CONCLUSIONS
In this study, it was found that patients with urolithiasis displayed oxidative stress characterized by a TDH tendency towards the disulfide side, and an inadequate antioxidant response identified by a lower level of native thiol as compared with healthy controls.
Keyword
MeSH Terms
Figure
Reference
-
1. Türk C, Neisius A, Petrik A, Seitz C, Skolarikos A, Tepeler A, et al. European Association of Urology guidelines on urolithiasis [Internet]. Arnhem: The European Association of Urology;2017. 3. cited 2019 Jan. Available from: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urolithiasis_2017_10-05V2.pdf.2. Yasui T, Okada A, Hamamoto S, Ando R, Taguchi K, Tozawa K, et al. Pathophysiology-based treatment of urolithiasis. Int J Urol. 2017; 24:32–38. PMID: 27539983.
Article3. Kaya E, Ebiloğlu T, Zor M, Yalççn S, Coğuplugil AE, Bedir S. The outcome of percutaneous nephrolithotomy on ≥50 mm staghorn and multiple calyceal stones. Turk J Urol. 2018; 44:148–152. PMID: 29511585.
Article4. Noureldin YA, da Silva A, Fahmy N, Andonian S. Is it safe to prescribe ascorbic acid for urinary acidification in stone-forming patients with alkaline urine? Turk J Urol. 2017; 43:183–188. PMID: 28717544.
Article5. Ceban E, Banov P, Galescu A, Botnari V. Oxidative stress and antioxidant status in patients with complicated urolithiasis. J Med Life. 2016; 9:259–262. PMID: 27974930.6. Ma MC, Chen YS, Huang HS. Erythrocyte oxidative stress in patients with calcium oxalate stones correlates with stone size and renal tubular damage. Urology. 2014; 83:510.e9–510.e17.
Article7. Engin A, Altan N. Effects of obstructive jaundice on the antioxidative capacity of human red blood cells. Haematologia (Budap). 2000; 30:91–96. PMID: 10839561.
Article8. Yardim-Akaydin S, Sepici A, Ozkan Y, Torun M, Simşek B, Sepici V. Oxidation of uric acid in rheumatoid arthritis: is allantoin a marker of oxidative stress. Free Radic Res. 2004; 38:623–628. PMID: 15346653.
Article9. Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001; 477:7–21. PMID: 11376682.
Article10. Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013; 288:26489–26496. PMID: 23861395.
Article11. Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000; 72(2 Suppl):653S–669S. PMID: 10919972.
Article12. Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014; 47:326–332. PMID: 25304913.
Article13. Altıparmak IH, Erkuş ME, Sezen H, Demirbag R, Gunebakmaz O, Kaya Z, et al. The relation of serum thiol levels and thiol/disulphide homeostasis with the severity of coronary artery disease. Kardiol Pol. 2016; 74:1346–1353. PMID: 27221962.
Article14. Ates I, Kaplan M, Yuksel M, Mese D, Alisik M, Erel Ö, et al. Determination of thiol/disulphide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine. 2016; 51:47–51. PMID: 26547218.
Article15. Dirican N, Dirican A, Sen O, Aynali A, Atalay S, Bircan HA, et al. Thiol/disulfide homeostasis: a prognostic biomarker for patients with advanced non-small cell lung cancer? Redox Rep. 2016; 21:197–203. PMID: 26200761.
Article16. Göknar N, Oktem F, Arı E, Demir AD, Torun E. Is oxidative stress related to childhood urolithiasis? Pediatr Nephrol. 2014; 29:1381–1386. PMID: 24526098.
Article17. Khan SR. Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res. 2006; 34:86–91. PMID: 16404622.
Article18. Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012; 40:95–112. PMID: 22213019.
Article19. Fishman AI, Green D, Lynch A, Choudhury M, Eshghi M, Konno S. Preventive effect of specific antioxidant on oxidative renal cell injury associated with renal crystal formation. Urology. 2013; 82:489.e1–489.e7.
Article20. Yücel D. C-Reactive protein vs. high-sensitivity C-reactive protein: what is the difference? Turk J Biochem. 2014; 39:43–44.21. Seropian IM, Romeo FJ, Pizarro R, Vulcano NO, Posatini RA, Marenchino RG, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictors of survival after heart transplantation. ESC Heart Fail. 2018; 5:149–156. PMID: 28758719.
Article22. Hirose M, Yasui T, Okada A, Hamamoto S, Shimizu H, Itoh Y, et al. Renal tubular epithelial cell injury and oxidative stress induce calcium oxalate crystal formation in mouse kidney. Int J Urol. 2010; 17:83–92. PMID: 19919640.
Article23. Hirose M, Tozawa K, Okada A, Hamamoto S, Higashibata Y, Gao B, et al. Role of osteopontin in early phase of renal crystal formation: immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urol Res. 2012; 40:121–129. PMID: 21833789.
Article24. Grases F, Costa-Bauzá A, Bonarriba CR, Pieras EC, Fernández RA, Rodríguez A. On the origin of calcium oxalate monohydrate papillary renal stones. Urolithiasis. 2015; 43(Suppl 1):33–39. PMID: 25086903.
Article25. Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol. 2005; 173:271–275. PMID: 15592095.
Article26. Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Examination of the anti-oxidative effect in renal tubular cells and apoptosis by oxidative stress. Urol Res. 2005; 33:261–266. PMID: 16047215.
Article27. Bal C, Büyükşekerci M, Koca C, AXğış ER, Erdoğan S, Baran P, et al. The compromise of dynamic disulfide/thiol homeostasis as a biomarker of oxidative stress in trichloroethylene exposure. Hum Exp Toxicol. 2016; 35:915–920. PMID: 26429930.
Article28. Emre S, Demirseren DD, Alisik M, Aktas A, Neselioglu S, Erel O. Dynamic thiol/disulfide homeostasis and effects of smoking on homeostasis parameters in patients with psoriasis. Cutan Ocul Toxicol. 2017; 36:393–396. PMID: 28397526.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Evaluation of Thiol/Disulfide Homeostasis and Oxidative DNA Damage in Patients with Obsessive Compulsive Disorder
- Dynamic Thiol/Disulfide Homeostasis and Oxidative DNA Damage in Adult Attention Deficit Hyperactivity Disorder
- Thiol Disulfide Homeostasis in Schizophrenic Patients Using Atypical Antipsychotic Drugs
- Thiol/Disulfide Homeostasis in Bipolar and Unipolar Depression
- Thiol-disulphide Homeostasis in Patients with Schizophrenia: The Potential Biomarkers of Oxidative Stress in Acute Exacerbation of Schizophrenia