Yonsei Med J.
2019 Jul;60(7):611-618. 10.3349/ymj.2019.60.7.611.
Family with Sequence Similarity 83 Member H Promotes the Viability and Metastasis of Cervical Cancer Cells and Indicates a Poor Prognosis
- Affiliations
-
- 1Department of Radiotherapy, Zhejiang Provincial Hospital of Traditional Chinese Medicine, Hangzhou, Zhejiang, China.
- 2Department of Geratology, Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou, Zhejiang, China. zhoujia_1122@163.com
- KMID: 2450400
- DOI: http://doi.org/10.3349/ymj.2019.60.7.611
Abstract
- PURPOSE
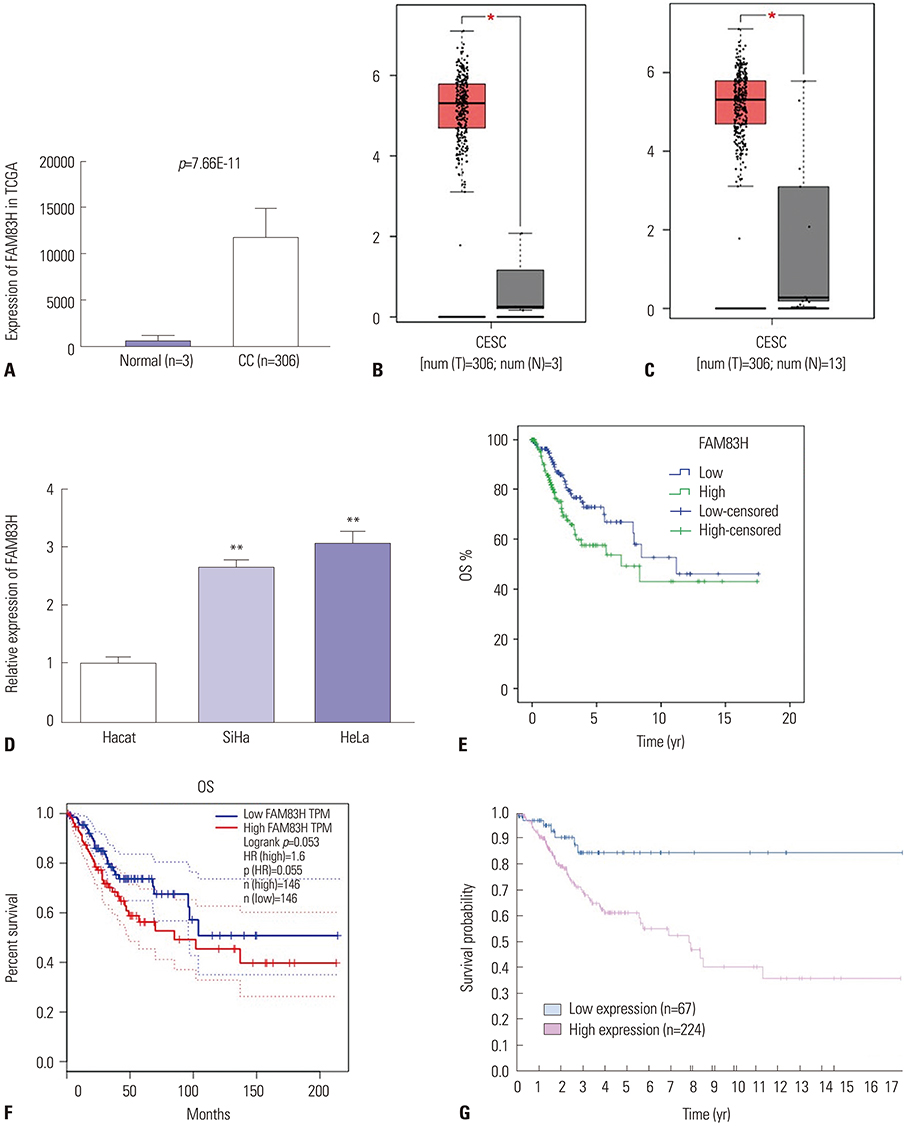
Family with sequence similarity 83 member H (FAM83H) plays key roles in tumorigenesis. However, the specific roles of FAM83H in cervical cancer (CC) have not been well studied.
MATERIALS AND METHODS
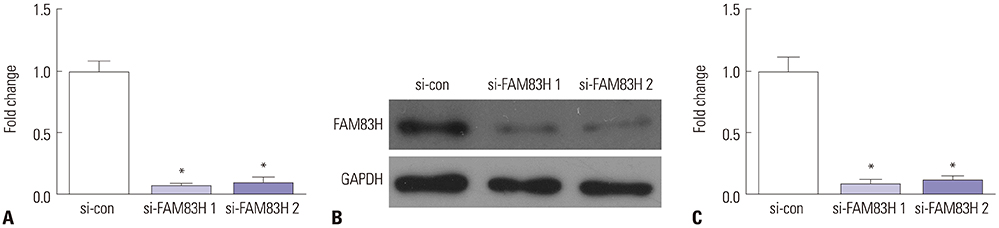
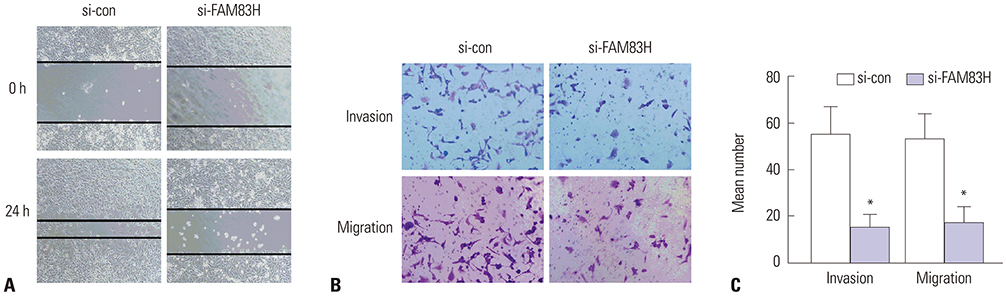
The RNA-seq data of 306 CC tissues and three normal samples downloaded from The Cancer Genome Atlas were used to analyze the expression of FAM83H. The Kaplan-Meier method was used to draw survival curves. Associations between FAM83H expression and clinicopathological factors were analyzed by chi-square test. Cox proportional hazards model was used to analyze prognostic factors. Loss-of-function assays were conducted to discover the biological functions of FAM83H in cell proliferation, colony formation, invasion, and migration. Real-time Quantitative Reverse Transcription PCR (qRT-PCR) and Western blotting were used to measure the expression levels of FAM83H in CC cell lines.
RESULTS
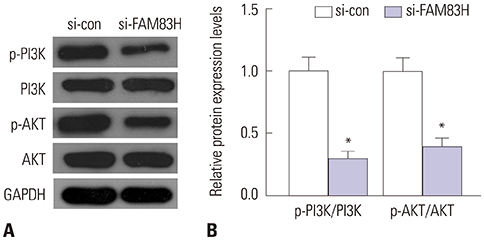
Our results demonstrated that FAM83H is overexpressed in CC tissues and that high FAM83H expression is associated with worse overall survival (OS). High FAM83H expression in CC was associated with clinical stage, pathologic tumor, and pathologic node. Univariate analysis suggested that FAM83H expression was significantly related to the OS of CC patients. Although multivariate analysis showed that FAM83H expression was not an independent prognostic factor for the OS of CC patients, the effects of FAM83H on CC cell growth and motility was significant. Loss-of-function experiments demonstrated that knockdown of FAM83H inhibited proliferation, colony formation, migration, and invasion of CC cells by inactivating PI3K/AKT pathway.
CONCLUSION
FAM83H might play a crucial role in CC progression and could act as a novel therapeutic target in CC.
Keyword
MeSH Terms
Figure
Reference
-
1. Ili CG, Brebi P, López J, García P, Leal P, Suarez E, et al. Genotyping of human papillomavirus in cervical intraepithelial neoplasia in a high-risk population. J Med Virol. 2011; 83:833–837.
Article2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–132.
Article3. Lee SY, Meier R, Furuta S, Lenburg ME, Kenny PA, Xu R, et al. FAM83A confers EGFR-TKI resistance in breast cancer cells and in mice. J Clin Invest. 2012; 122:3211–3220.
Article4. Wang Z, Liu Y, Zhang P, Zhang W, Wang W, Curr K, et al. FAM83D promotes cell proliferation and motility by downregulating tumor suppressor gene FBXW7. Oncotarget. 2013; 4:2476–2486.
Article5. Cipriano R, Graham J, Miskimen KL, Bryson BL, Bruntz RC, Scott SA, et al. FAM83B mediates EGFR- and RAS-driven oncogenic transformation. J Clin Invest. 2012; 122:3197–3210.
Article6. Snijders AM, Lee SY, Hang B, Hao W, Bissell MJ, Mao JH. FAM83 family oncogenes are broadly involved in human cancers: an integrative multi-omics approach. Mol Oncol. 2017; 11:167–179.
Article7. Kuga T, Sasaki M, Mikami T, Miake Y, Adachi J, Shimizu M, et al. FAM83H and casein kinase I regulate the organization of the keratin cytoskeleton and formation of desmosomes. Sci Rep. 2016; 6:26557.
Article8. Kuga T, Kume H, Kawasaki N, Sato M, Adachi J, Shiromizu T, et al. A novel mechanism of keratin cytoskeleton organization through casein kinase Iα and FAM83H in colorectal cancer. J Cell Sci. 2013; 126(Pt 20):4721–4731.
Article9. Kuga T, Kume H, Adachi J, Kawasaki N, Shimizu M, Hoshino I, et al. Casein kinase 1 is recruited to nuclear speckles by FAM83H and SON. Sci Rep. 2016; 6:34472.
Article10. Nalla AK, Williams TF, Collins CP, Rae DT, Trobridge GD. Lentiviral vector-mediated insertional mutagenesis screen identifies genes that influence androgen independent prostate cancer progression and predict clinical outcome. Mol Carcinog. 2016; 55:1761–1771.
Article11. Kim KM, Park SH, Bae JS, Noh SJ, Tao GZ, Kim JR, et al. FAM83H is involved in the progression of hepatocellular carcinoma and is regulated by MYC. Sci Rep. 2017; 7:3274.
Article12. Li L, Wang L, Song P, Geng X, Liang X, Zhou M, et al. Critical role of histone demethylase RBP2 in human gastric cancer angiogenesis. Mol Cancer. 2014; 13:81.
Article13. Jiang CG, Lv L, Liu FR, Wang ZN, Liu FN, Li YS, et al. Downregulation of connective tissue growth factor inhibits the growth and invasion of gastric cancer cells and attenuates peritoneal dissemination. Mol Cancer. 2011; 10:122.
Article14. Okano J, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, et al. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2003; 309:298–304.
Article15. Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, et al. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L354–L363.
Article16. Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006; 18:2262–2271.
Article17. Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007; 28:1379–1386.
Article18. Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999; 253:210–229.
Article19. Kim MJ, Lee TH, Kim SH, Choi YJ, Heo J, Kim YH. Triptolide inactivates Akt and induces caspase-dependent death in cervical cancer cells via the mitochondrial pathway. Int J Oncol. 2010; 37:1177–1185.
Article20. Kim TJ, Lee JW, Song SY, Choi JJ, Choi CH, Kim BG, et al. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer. 2006; 94:1678–1682.
Article21. Bertelsen BI, Steine SJ, Sandvei R, Molven A, Laerum OD. Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: frequent PIK3CA amplification and AKT phosphorylation. Int J Cancer. 2006; 118:1877–1883.
Article22. Liu SC, Chen C, Chung CH, Wang PC, Wu NL, Cheng JK, et al. Inhibitory effects of butein on cancer metastasis and bioenergetic modulation. J Agric Food Chem. 2014; 62:9109–9117.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metastatic Breast Cancer from Cervical Cancer
- The Cancer/Testis Antigen CT45A1 Promotes Transcription of Oncogenic Sulfatase-2 Gene in Breast Cancer Cells and Is Sensible Targets for Cancer Therapy
- Expression of CD44v6 in Cervical Cancer
- A Case of Skin Metastasis from Uterine Cervical Cancer
- Cloning of BNIP3h, a member of proapoptotic BNIP3 family genes