Intest Res.
2019 Apr;17(2):237-243. 10.5217/ir.2018.00071.
Influence of anti-tumor necrosis factor-alpha therapy to pregnant inflammatory bowel disease women and their children's immunity
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea. jassa@ewha.ac.kr
- 2Department of Gastroenterology, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 5Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 2449965
- DOI: http://doi.org/10.5217/ir.2018.00071
Abstract
- BACKGROUND/AIMS
The onset of inflammatory bowel disease (IBD) usually occurs at young age, and therefore, women IBD patients experience pregnancy during their disease progression. Recently, the use of anti-tumor necrosis factor-α (anti-TNF-α) has been rapidly increasing. The aim of this study was to evaluate pregnancy related outcomes in women with IBD who were treated with anti-TNF-α during pregnancy and immunity of their children.
METHODS
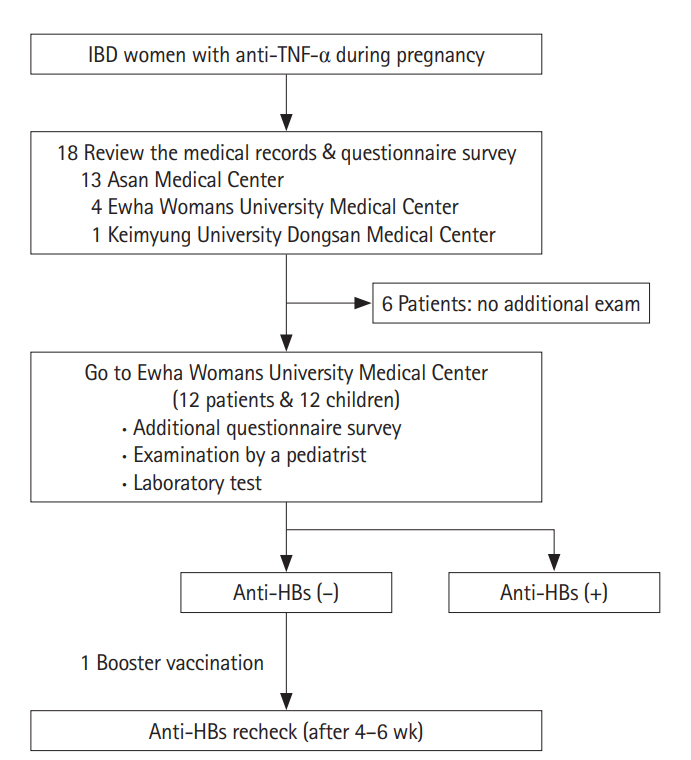
Korean women with IBD who had been treated with anti-TNF-α during pregnancy had been enrolled. Medical records were reviewed and a survey was performed for each patient. For the patients who agreed on additional examination for their children, children's growth, medical history and antibody to hepatitis B surface antigen (anti-HBs) titer were checked.
RESULTS
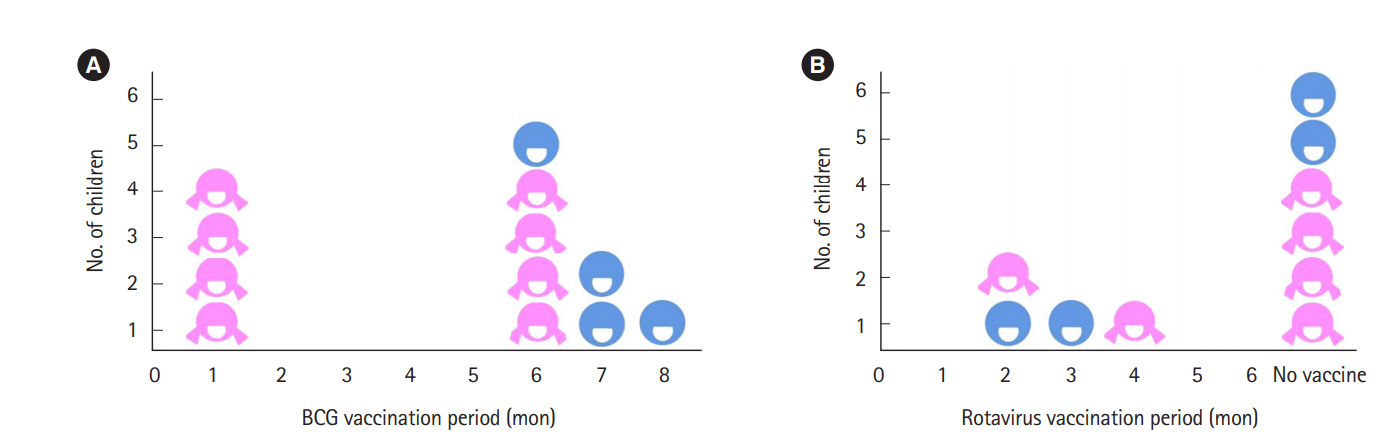
All 18 patients had been diagnosed with Crohn's disease. There was not any case of preterm delivery, low birth-weight infant, congenital anomaly, nor stillbirth. All 12 children had followed the regular vaccination schedule for hepatitis B and 4 of them showed negative results for anti-HBs. After the 1 booster vaccination, all children demonstrated seroconversion. Regarding live vaccines, 4 children had bacillus Calmette-Guerin and 4 had rotavirus vaccine before 6 months, without any specific side effects.
CONCLUSIONS
This was the first study of immunity of the children born from IBD women who had been treated with anti-TNF-α medication during their pregnancy. IBD women had comparable pregnancy outcomes with the general women population, suggesting that the disease activity rather than the administered medication would be more important in healthy pregnancy. Considering the history of vaccination and anti-HBs titers, immunity seems to be intact in the children.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Pregnancy Outcomes Associated With Biologic Agent Exposure in Patients With Several Rheumatic Diseases and Inflammatory Bowel Diseases
Soo Min Ahn, Young Bin Joo, Yun Jin Kim, So-Young Bang, Hye-Soon Lee
J Korean Med Sci. 2023;38(22):e172. doi: 10.3346/jkms.2023.38.e172.
Reference
-
1. Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002; 31:1–20.
Article2. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.
Article3. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.
Article4. Bush MC, Patel S, Lapinski RH, Stone JL. Perinatal outcomes in inflammatory bowel disease. J Matern Fetal Neonatal Med. 2004; 15:237–241.
Article5. Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004; 99:2385–2392.
Article6. Nørgård B, Hundborg HH, Jacobsen BA, Nielsen GL, Fonager K. Disease activity in pregnant women with Crohn’s disease and birth outcomes: a regional Danish cohort study. Am J Gastroenterol. 2007; 102:1947–1954.
Article7. Reddy D, Murphy SJ, Kane SV, Present DH, Kornbluth AA. Relapses of inflammatory bowel disease during pregnancy: inhospital management and birth outcomes. Am J Gastroenterol. 2008; 103:1203–1209.
Article8. Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011; 106:214–223.
Article9. Schulze H, Esters P, Dignass A. Review article: the management of Crohn’s disease and ulcerative colitis during pregnancy and lactation. Aliment Pharmacol Ther. 2014; 40:991–1008.
Article10. Ng SW, Mahadevan U. My treatment approach to management of the pregnant patient with inflammatory bowel disease. Mayo Clin Proc. 2014; 89:355–360.
Article11. McConnell RA, Mahadevan U. Pregnancy and the patient with inflammatory bowel disease: fertility, treatment, delivery, and complications. Gastroenterol Clin North Am. 2016; 45:285–301.12. Morales M, Berney T, Jenny A, Morel P, Extermann P. Crohn’s disease as a risk factor for the outcome of pregnancy. Hepatogastroenterology. 2000; 47:1595–1598.13. Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002; 97:641–648.
Article14. Mottet C, Juillerat P, Pittet V, et al. Pregnancy and breastfeeding in patients with Crohn’s disease. Digestion. 2007; 76:149–160.
Article15. Ferguson CB, Mahsud-Dornan S, Patterson RN. Inflammatory bowel disease in pregnancy. BMJ. 2008; 337:a427.
Article16. Vermeire S, Carbonnel F, Coulie PG, et al. Management of inflammatory bowel disease in pregnancy. J Crohns Colitis. 2012; 6:811–823.
Article17. van der Woude CJ, Kolacek S, Dotan I, et al. European evidenced-based consensus on reproduction in inflammatory bowel disease. J Crohns Colitis. 2010; 4:493–510.
Article18. Dretzke J, Edlin R, Round J, et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-alpha) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol Assess. 2011; 15:1–244.19. D’Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J astroenterol. 2011; 106:199–212.
Article20. Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final rule. Fed Regist. 2014; 79:72063–72103.21. Department of Health Therapeutic Goods Administration. Australian categorisation system for prescribing medicines in pregnancy. Australian Government Web site. https://www.tga.gov.au/australian-categorisation-system-prescribing-medicines-pregnancy. Updated May 4, 2011. Accessed November 26, 2018.22. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996; 36:248–255.
Article23. Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol. 2009; 104:228–233.
Article24. Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003; 21:3365–3369.
Article25. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006; 4:621–630.
Article26. Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013; 11:286–292.
Article27. Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006; 4:1255–1258.
Article28. Zelinkova Z, de Haar C, de Ridder L, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011; 33:1053–1058.
Article29. Carter JD, Ladhani A, Ricca LR, Valeriano J, Vasey FB. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009; 36:635–641.
Article30. El Mourabet M, El-Hachem S, Harrison JR, Binion DG. Anti-TNF antibody therapy for inflammatory bowel disease during pregnancy: a clinical review. Curr Drug Targets. 2010; 11:234–241.
Article31. Gisbert JP. Safety of immunomodulators and biologics for the treatment of inflammatory bowel disease during pregnancy and breast-feeding. Inflamm Bowel Dis. 2010; 16:881–895.
Article32. Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy. Gastroenterology. 2012; 142:S–149.33. Julsgaard M, Christensen LA, Gibson PR, et al. Adalimumab and infliximab level in neonates. Gastroenterology. 2015; 148:S–108.34. KOSIS. Review & assessment results for delivery: classification by birth weight. Statistics Korea Web site. http://kostat.go.kr/portal/korea/index.action. Accessed November 26, 2018.35. An YW, Chung EH, Rheem I. A study of the current (2003-2005) prevalence of anti-HBs and immunologic memory of hepatitis B vaccine in children from the central area of Korea. Korean J Pediatr. 2006; 49:630–634.
Article36. Sheibani S, Cohen R, Kane S, Dubinsky M, Church JA, Mahadevan U. The effect of maternal peripartum anti-TNFalpha use on infant immune response. Dig Dis Sci. 2016; 61:1622–1627.
Article37. Beaulieu DB, Ananthakrishnan AN, Martin C, Cohen RD, Kane SV, Mahadevan U. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol. 2018; 16:99–105.
Article38. Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013; 108:1426–1438.
Article39. Bortlik M, Duricova D, Machkova N, et al. Impact of anti-tumor necrosis factor alpha antibodies administered to pregnant women with inflammatory bowel disease on long-term outcome of exposed children. Inflamm Bowel Dis. 2014; 20:495–501.
Article40. Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010; 4:603–605.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biological Therapy for Inflammatory Bowel Disease in Children
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis
- Reactivation of Hepatitis B Virus Following Anti-Tumor Necrosis Factor-alpha Therapy
- Is the Therapeutic Drug Monitoring of Anti-TNF Agents Necessary in Korean Inflammatory Bowel Disease Patients?
- Effect of Immunomodulators and Biologic Agents on Malignancy in Patients with Inflammatory Bowel Disease