Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn's disease?: a systematic review
- Affiliations
-
- 1Nottingham Digestive Diseases Centre, University of Nottingham, Nottingham, UK. Gordon.Moran@nottingham. ac.uk
- 2National Institute of Health Research, Nottingham Biomedical Research Centre, Nottingham University Hospitals and the University of Nottingham, Nottingham, UK.
- 3Department of Gastroenterology, Christian Medical College, Vellore, India.
- 4Libraries, Research & Learning Resources, University of Nottingham, Nottingham, UK.
- KMID: 2449957
- DOI: http://doi.org/10.5217/ir.2018.00114
Abstract
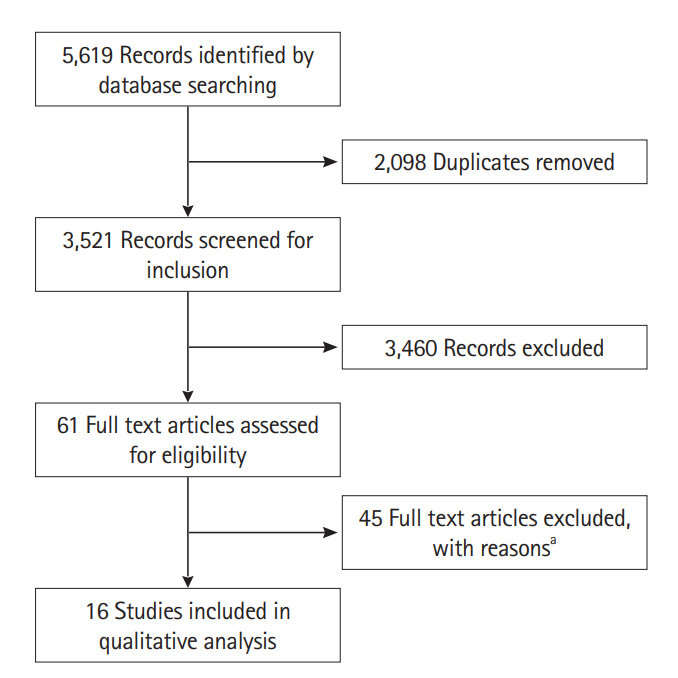
- Fecal calprotectin (FC) is a highly sensitive disease activity biomarker in inflammatory bowel disease. However, there are conflicting reports on whether the diagnostic accuracy in Crohn's disease is influenced by disease location. The aim of this study was to undertake a systematic review of the published literature. Relevant databases were searched from inception to November 8, 2016 for cohort and case control studies which had data on FC in patients with isolated small bowel (SB) and large bowel (LB) Crohn's disease. Reference standards for disease activity were endoscopy, magnetic resonance imaging, computed tomography or a combination of these. The QUADAS-2 research tool was used to assess the risk of bias. There were 5,619 records identified at initial search. The 2,098 duplicates were removed and 3,521 records screened. Sixty-one full text articles were assessed for eligibility and 16 studies were included in the final review with sensitivities and specificities per disease location available from 8 studies. Sensitivities of FC at SB and LB locations ranged from 42.9% to 100% and 66.7% to 100% respectively while corresponding specificities were 50% to 100% and 28.6% to 100% respectively. The sensitivities and specificities of FC to accurately measure disease activity in Crohn's disease at different disease locations are diverse and no firm conclusion can be made. Better studies need to be undertaken to categorically answer the effect of disease location on the diagnostic accuracy of FC.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Fecal S100A12 is associated with future hospitalization and step-up of medical treatment in patients with Crohn’s disease in clinical remission: a pilot study
Sun-Ho Lee, Sung Wook Hwang, Sang Hyoung Park, Dong-Hoon Yang, Jeong-Sik Byeon, Seung-Jae Myung, Suk-Kyun Yang, Byong Duk Ye
Intest Res. 2022;20(2):203-212. doi: 10.5217/ir.2021.00020.Intestinal ultrasonography and fecal calprotectin for monitoring inflammation of ileal Crohn’s disease: two complementary tests
José María Paredes, Tomás Ripollés, Ángela Algarra, Rafael Diaz, Nadia Moreno, Patricia Latorre, María Jesús Martínez, Pilar Llopis, Antonio López, Eduardo Moreno-Osset
Intest Res. 2022;20(3):361-369. doi: 10.5217/ir.2021.00126.Combination of leucine-rich alpha-2 glycoprotein and fecal markers detect Crohn’s disease activity confirmed by balloon-assisted enteroscopy
Ami Kawamoto, Kento Takenaka, Shuji Hibiya, Yoshio Kitazume, Hiromichi Shimizu, Toshimitsu Fujii, Eiko Saito, Kazuo Ohtsuka, Ryuichi Okamoto
Intest Res. 2024;22(1):65-74. doi: 10.5217/ir.2023.00092.Which biomarkers best reflect the degree of inflammation in Crohn’s disease?
Jihye Park
Intest Res. 2024;22(1):1-2. doi: 10.5217/ir.2023.00161.
Reference
-
1. Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984; 25:665–672.2. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990; 99:956–963.
Article3. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.
Article4. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009; 15:1295–1301.
Article5. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014; 12:414–422.
Article6. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010; 138:463–468.
Article7. Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002; 97:947–953.
Article8. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013; 7:982–1018.
Article9. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:802–819.
Article10. Lehmann FS, Burri E, Beglinger C. The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol. 2015; 8:23–36.
Article11. Sipponen T, Savilahti E, Karkkainen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008; 14:1392–1398.
Article12. Gisbert JP, Bermejo F, Pérez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009; 15:1190–1198.
Article13. D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008; 103:2007–2014.
Article14. af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012; 47:528–537.
Article15. Inoue K, Aomatsu T, Yoden A, Okuhira T, Kaji E, Tamai H. Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel diseases. J Gastroenterol Hepatol. 2014; 29:1406–1412.
Article16. D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007; 22:429–437.
Article17. Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015; 50:841–847.
Article18. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008; 14:40–46.
Article19. Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011; 46:694–700.
Article20. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013; 7:556–585.
Article21. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536.
Article22. Astle VI, Lewis NR. PWE-116 Association of faecal calprotectin with extent and distribution of inflammation in IBD. Gut. 2014; 63(Suppl 1):A175–A176.23. Bodelier A, de Boer E, Jonkers D, Hameeteman W, Masclee A, Pierik MJ. Monitoring disease activity in IBD: correlation between clinical activity indices and biomarkers. Gastroenterology. 2011; 140(5):S–423.
Article24. Bojic D, Bojic B, Protic M, Smith K. Fecal calprotectin is reliable surrogate marker of endoscopic and histologic mucosal healing in Crohn’s disease and ulcerative colitis. J Crohns Colitis. 2011; 5:S34.25. Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008; 40:547–553.
Article26. Cerrillo E, Beltrán B, Pous S, et al. Fecal calprotectin in ileal Crohn’s disease: relationship with magnetic resonance enterography and a pathology score. Inflamm Bowel Dis. 2015; 21:1572–1579.27. Chang J, Kennedy NA, Spurio FF, et al. Tu1132 Correlation of clinical symptoms to current biomarkers of intestinal inflammation in patients with Crohn’s disease. Gastroenterology. 2013; 144(5 Suppl 1):S–770.
Article28. Chung-Faye G, Sandhu K, Logan RP, Sherwood RA. Fecal calproctectin is strongly predictive of clinical disease activity and histological severity in inflammatory bowel disease. Gastroenterology. 2011; 140(5 Suppl 1):S–421.
Article29. Costa F, Mumolo MG, Bellini M, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003; 35:642–647.
Article30. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.31. D’Haens GR, Baert F, Vermeire S, et al. Mucosal inflammation in inflammatory bowel disease can reliably be predicted with the fecal calprotectin test. Gastroenterology. 2007; 132(4 Supp 2):A174.32. Dranga M, Dumitrescu G, Badea M, Blaj A, Mihai C, Prelipcean CC. The semi-quantitative calprotectin rapid test: is it useful in inflammatory bowel disease? Rev Med Chir Soc Med Nat Iasi. 2012; 116:761–765.33. Gaya DR, Duncan A, Lyon TD, et al. 343 Faecal calprotectin: a non-invasive, sensitive, and objective method in the assessment of Crohn’s disease activity. Gut. 2005; 54:A90.34. Gerasimidis K, Nikolaou CK, Edwards CA, McGrogan P. Serial fecal calprotectin changes in children with Crohn’s disease on treatment with exclusive enteral nutrition: associations with disease activity, treatment response, and prediction of a clinical relapse. J Clin Gastroenterol. 2011; 45:234–239.
Article35. Inokuchi T, Kato J, Hiraoka S, et al. Su1248 Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn’s disease. Gastroenterology. 2015; 148(4 Suppl 1):S–451.
Article36. Kennedy NA, Clark A, Walkden A, et al. Clinical utility and diagnostic accuracy of faecal calprotectin for IBD at first presentation to gastroenterology services in adults aged 16-50 years. J Crohns Colitis. 2015; 9:41–49.37. Laharie D, Mesli S, El Hajbi F, et al. Prediction of Crohn’s disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. 2011; 34:462–469.
Article38. Makanyanga J, Pendse D, Atkins E, et al. PWE-231 MRI is correlated to faecal calprotectin level in the evaluation of small bowel and colonic Crohn’s disease. Gut. 2012; 61(Suppl 2):A392.39. Minar P, Jurickova I, Haberman Y, et al. Tu1940 Neutrophil FCy receptor 1 (CD64) index as a non-invasive biomarker for clinical and mucosal disease activity in pediatric inflammatory bowel disease. Gastroenterology. 2013; 144(5 Suppl 1):S–886.
Article40. Naismith GD, Smith LA, Barry SJ, et al. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn’s disease. J Crohns Colitis. 2014; 8:1022–1029.
Article41. Nikolaus S, Schreiber S, Nurwakagari P, Rath S, Wittig BM, Schwarz M. Su1279 Clinical epidemiology of fecal calprotectin: population data from the fire study, a prospective longitudinal study in germany to evaluate fecal calprotectin in routine monitoring of Crohn’s disease. Gastroenterology. 2014; 146(5 Suppl 1):S–423.
Article42. Palmon R, Brown S, Ullman TA, Hanaway P, Mayer LF. Calprotectin and lactoferrin decrease with maintenance infliximab administration in luminal Crohn’s disease. Gastroenterology. 2006; 130(4 Suppl 2):A212–A213.43. Pavlidis P, Cavazza A, Siddique N, et al. PTH-059 Faecal calprotectin identifies non responders to anti-TNFalpha therapy when measured after induction in inflammatory Crohn’s disease. Gut. 2015; 64(Suppl 1):A431–A432.44. Scaioli E, Cardamone C, Scagliarini M, Zagari RM, Bazzoli F, Belluzzi A. Can fecal calprotectin better stratify Crohn’s disease activity index? Ann Gastroenterol. 2015; 28:247–252.45. Shah R, Herrera H, Dewald R, Swaroop P. Predictors of elevated fecal calprotectin in inflammatory bowel disease patients: P-85. Inflamm Bowel Dis. 2011; 17(Suppl 2):S39–S40.46. Tursi A, Elisei W, Giorgetti G, Picchio M, Brandimarte G. Rapid fecal calprotectin correlates with clinical and endoscopic severity of inflammatory bowel diseases. Scand J Gastroenterol. 2013; 48:1359–1360.
Article47. Turvill J. Mapping of Crohn’s disease outcomes to faecal calprotectin levels in patients maintained on biologic therapy. Frontline Gastroenterol. 2014; 5:167–175.
Article48. Zubin G, Peter L. Predicting endoscopic Crohn’s disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis. 2015; 21:1386–1391.49. Schröder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther. 2007; 26:1035–1042.
Article50. Warner BD, Johnston EL, Ward MG, Irving PM. PTU-076 Is faecal calprotectin (FC) a reliable marker of isolated small bowel Crohn’s disease (CD) activity? Gut. 2015; 64(Suppl 1):A93–A94.51. Bremner A, Roked S, Robinson R, Phillips I, Beattie M. Faecal calprotectin in children with chronic gastrointestinal symptoms. Acta Paediatr. 2005; 94:1855–1858.
Article52. Shaoul R, Sladek M, Turner D, et al. Limitations of fecal calprotectin at diagnosis in untreated pediatric Crohn’s disease. Inflamm Bowel Dis. 2012; 18:1493–1497.
Article53. Komraus M, Wos H, Wiecek S, Kajor M, Grzybowska-Chlebowczyk U. Usefulness of faecal calprotectin measurement in children with various types of inflammatory bowel disease. Mediators Inflamm. 2012; 2012:608249.
Article54. Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014; 109:637–645.
Article55. Dobrzanski C, Pedersen N, Burisch J, Hansen VV, Fuglsang H, Munkholm P. P643 Faecal calprotectin: correlation with the Harvey–Bradshaw Index in patients with Crohn’s disease. J Crohns Colitis. 2013; 7(Suppl 1):S268.
Article56. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000; 119:15–22.
Article57. García-Sánchez V, Iglesias-Flores E, González R, et al. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010; 4:144–152.
Article58. Diamanti A, Colistro F, Basso MS, et al. Clinical role of calprotectin assay in determining histological relapses in children affected by inflammatory bowel diseases. Inflamm Bowel Dis. 2008; 14:1229–1235.
Article59. Meunier P, Cousin F, Van Kemseke C, et al. Persisting signs of disease activity at magnetic resonance enterocolonography predict clinical relapse and disease progression in quiescent Crohn’s disease. Acta Gastroenterol Belg. 2015; 78:274–281.60. Lin WC, Wong JM, Lin CP, et al. Fecal calprotectin levels could be used as a predictor of endoscopic remission for inflammatory bowel disease patients: Taiwan Society of Inflammatory Bowel Disease Multicenter Study. Clin Gastroenterol Hepatol. 2015; 13:e101.
Article61. Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001; 33:14–22.
Article62. Guidi L, Marzo M, Andrisani G, et al. Faecal calprotectin assay after induction with anti-tumour necrosis factor alpha agents in inflammatory bowel disease: prediction of clinical response and mucosal healing at one year. Dig Liver Dis. 2014; 46:974–979.
Article63. Tang J, Gao X, Zhi M, Hu P. P101 Fecal calprotectin is a valuable marker for detecting active Crohns disease with colon involvement. J Crohn Colitis. 2015; 9(Suppl 1):S127.64. Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008; 6:1218–1224.
Article65. Faubion WA, Fletcher JG, de Villiers WJ, et al. Mo1680 Assessing Crohn’s disease inflammatory biomarker diagnostic accuracy using ileocolonoscopy or a combined ileocolonoscopy-CTE score in the Embark Study. Gastroenterology. 2012; 142(Suppl 1):S–658.
Article66. Faubion WA Jr, Fletcher JG, O’Byrne S, et al. EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013; 108:1891–1900.
Article67. Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013; 7:e641–e651.
Article68. Makanyanga JC, Pendsé D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014; 24:277–287.
Article69. Maltz B, Milne G, Slaughter JC, Merchant N, Schwartz DA. W1153 The utility of urinary prostaglandin E-M (PGE-M) as a biomarker of Crohn’s disease activity. Gastroenterology. 2009; 136(5 Suppl 1):A–665.
Article70. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s Disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010; 105:162–169.
Article71. Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008; 28:1221–1229.
Article72. Stawczyk-Eder K, Eder P, Lykowska-Szuber L, et al. Is faecal calprotectin equally useful in all Crohn’s disease locations? A prospective, comparative study. Arch Med Sci. 2015; 11:353–361.
Article73. Zittan E, Kelly O, Burns J, et al. Su1236 High fecal calprotectin correlate with active colonic disease but not with small intestinal Crohn’s disease activity. Gastroenterology. 2015; 148(4 Suppl 1):S–448.
Article74. Moniuszko A, Koziel D, Gluszek S, Rydzewska G. P244 Is prognostic utility of rapid faecal calprotectin test equal in all inflammatory bowel disease (IBD) subtypes? Retrospective analysis based on endoscopic indices. J Crohns Colitis. 2016; 10(Suppl 1):S216.75. Goutorbe F, Goutte M, Minet-Quinard R, et al. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J Crohns Colitis. 2015; 9:1113–1119.
Article76. Lin WC, Wong JM, Tung CC, et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015; 21:13566–13573.
Article77. García-Bosch O, Ordás I, Aceituno M, et al. Comparison of diagnostic accuracy and impact of magnetic resonance imaging and colonoscopy for the management of Crohn’s disease. J Crohns Colitis. 2016; 10:663–669.
Article78. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. National Institute for Health and Care Excellence (NICE) Web site. https://www.nice.org.uk/guidance/dg11. Accessed Aug 31,. 2017.79. Magro F, Lopes S, Coelho R, et al. Accuracy of faecal calprotectin and neutrophil gelatinase B-associated lipocalin in evaluating subclinical inflammation in UlceRaTIVE colitisthe ACERTIVE study. J Crohns Colitis. 2017; 11:435–444.
Article80. van Rheenen PF, Van de Vijver E, Fidler V, et al. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010; 341:c3369.
Article81. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390:2769–2778.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Calprotectin in Inflammatory Bowel Disease
- Diagnostic Accuracy of Fecal Calprotectin for the Detection of Small Bowel Crohn’s Disease through Capsule Endoscopy: An Updated Meta-Analysis and Systematic Review
- Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin?
- Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
- Combination of leucine-rich alpha-2 glycoprotein and fecal markers detect Crohn’s disease activity confirmed by balloon-assisted enteroscopy