Intest Res.
2016 Jan;14(1):5-14. 10.5217/ir.2016.14.1.5.
Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin?
- Affiliations
-
- 1Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan. katojun@wakayama-med.ac.jp
- 2Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
- KMID: 2174504
- DOI: http://doi.org/10.5217/ir.2016.14.1.5
Abstract
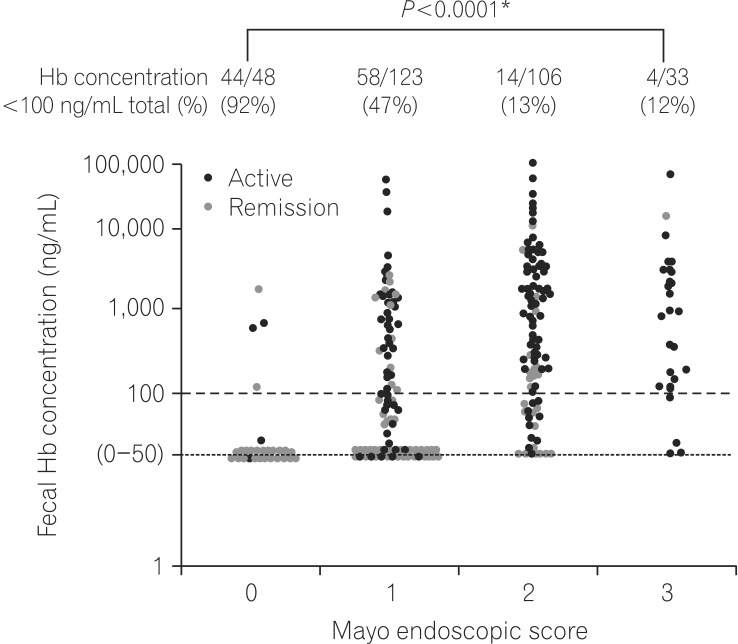
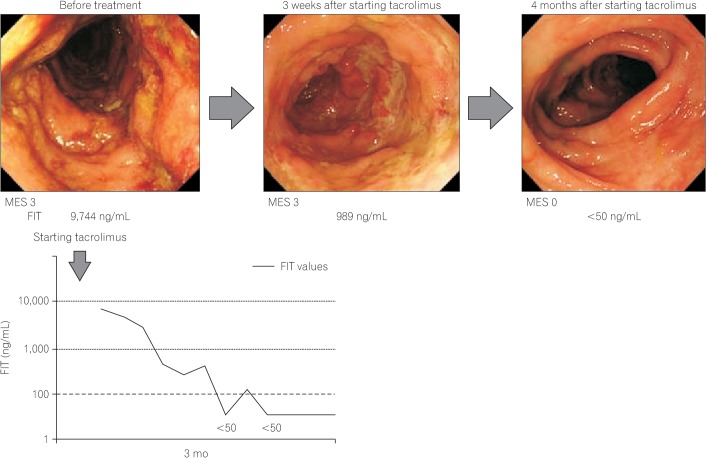
- Accurate evaluation of disease activity is essential for choosing an appropriate treatment and follow-up plan for patients with inflammatory bowel disease (IBD). Endoscopy is required for accurately evaluating disease activity, but the procedures are sometimes invasive and burdensome to patients. Therefore, alternative non-invasive methods for evaluating or predicting disease activity including mucosal status are desirable. Fecal calprotectin (Fcal) is the most widely used fecal marker for IBD, and many articles have described the performance of the marker in predicting disease activity, mucosal healing (MH), treatment efficacy, and risk of relapse. Fecal immunochemical test (FIT) can quantify the concentration of hemoglobin in stool and was originally used for the screening of colorectal cancer. We recently reported that FIT is also a useful biomarker for IBD. A direct comparison between the use of Fcal and FIT showed that both methods predicted MH in ulcerative colitis equally well. However, in the case of Crohn's disease, FIT was less sensitive to lesions in the small intestine, compared to Fcal. FIT holds several advantages over Fcal in regards to user-friendliness, including a lower cost, easy and clean handling, and the ability to make rapid measurements by using an automated measurement system. However, there is insufficient data to support the application of FIT in IBD. Further studies into the use of FIT for evaluating the inflammatory status of IBD are warranted.
MeSH Terms
Figure
Cited by 1 articles
-
Monitoring Disease Activity: How and When?
Kang-Moon Lee
Korean J Gastroenterol. 2018;71(2):69-73. doi: 10.4166/kjg.2018.71.2.69.
Reference
-
1. Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994; 35:231–235. PMID: 7508411.
Article2. Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity ininflammatory bowel disease. Inflamm Bowel Dis. 2005; 11:707–712. PMID: 16043984.
Article3. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002; 122:512–530. PMID: 11832465.
Article4. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994; 330:1841–1845. PMID: 8196726.
Article5. Irvine EJ. Usual therapy improves perianal Crohn's disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995; 20:27–32. PMID: 7884173.6. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999; 340:1398–1405. PMID: 10228190.
Article7. Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010; 7:15–29. PMID: 19949430.
Article8. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201. PMID: 21723220.
Article9. Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011; 9:483–489. PMID: 21195796.
Article10. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010; 138:463–468. PMID: 19818785.
Article11. Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006; 63:433–442. PMID: 16500392.
Article12. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009; 15:1295–1301. PMID: 19340881.
Article13. Frøslie KF, Jahnsen J, Moum BA, Vatn MH. IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422. PMID: 17681162.
Article14. Rutgeerts P, Vermeire S, Van Assche G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut. 2007; 56:453–455. PMID: 17369375.
Article15. D'Haens GR, Fedorak R, Lémann M, et al. Endpoints for clinical trials evaluating disease modification and structural damage in adults with Crohn's disease. Inflamm Bowel Dis. 2009; 15:1599–1604. PMID: 19653291.16. Menees S, Higgins P, Korsnes S, Elta G. Does colonoscopy cause increased ulcerative colitis symptoms? Inflamm Bowel Dis. 2007; 13:12–18. PMID: 17206634.
Article17. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005; 129:422–428. PMID: 16083699.
Article18. Faivre J, Dancourt V, Denis B, et al. Comparison between a guaiac and three immunochemical faecal occult blood tests in screening for colorectal cancer. Eur J Cancer. 2012; 48:2969–2976. PMID: 22572481.
Article19. Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013; 108:83–89. PMID: 23007005.
Article20. Inokuchi T, Kato J, Hiraoka S, et al. Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn's disease. Inflamm Bowel Dis. 2016; in press.
Article21. Haug U, Knudsen AB, Brenner H, Kuntz KM. Is fecal occult blood testing more sensitive for left-versus right-sided colorectal neoplasia? A systematic literature review. Expert Rev Mol Diagn. 2011; 11:605–616. PMID: 21745014.
Article22. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009; 15:1851–1858. PMID: 19462421.23. D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224. PMID: 22344983.24. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008; 14:40–46. PMID: 18022866.
Article25. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010; 105:162–169. PMID: 19755969.
Article26. Mooiweer E, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis. 2014; 20:307–314. PMID: 24374878.
Article27. Manes G, Imbesi V, Ardizzone S, et al. Appropriateness and diagnostic yield of colonoscopy in the management of patients with ulcerative colitis: a prospective study in an open access endoscopy service. Inflamm Bowel Dis. 2008; 14:1133–1138. PMID: 18314901.
Article28. Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard-Lassen I, Nielsen AM. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin Gastroenterol Hepatol. 2015; 13:1929–1936. PMID: 26051392.
Article29. Takashima S, Kato J, Hiraoka S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol. 2015; 110:873–880. PMID: 25823769.
Article30. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013; 19:2568–2576. PMID: 24013361.31. Meucci G, Fasoli R, Saibeni S, et al. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012; 18:1006–1010. PMID: 21830282.32. Laharie D, Filippi J, Roblin X, et al. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: a multicenter experience. Aliment Pharmacol Ther. 2013; 37:998–1004. PMID: 23521659.33. López-Palacios N, Mendoza JL, Taxonera C, Lana R, López-Jamar JM, Díaz-Rubio M. Mucosal healing for predicting clinical outcome in patients with ulcerative colitis using thiopurines in monotherapy. Eur J Intern Med. 2011; 22:621–625. PMID: 22075292.34. Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010; 16:338–346. PMID: 19637362.35. Nakarai A, Kato J, Hiraoka S, et al. Prognosis of ulcerative colitis differs between patients with complete and partial mucosal healing, which can be predicted from the platelet count. World J Gastroenterol. 2014; 20:18367–18374. PMID: 25561804.
Article36. Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008; 14:1392–1398. PMID: 18484671.
Article37. Kuriyama M, Kato J, Takemoto K, Hiraoka S, Okada H, Yamamoto K. Prediction of flare-ups of ulcerative colitis using quantitative immunochemical fecal occult blood test. World J Gastroenterol. 2010; 16:1110–1114. PMID: 20205282.
Article38. D'Incá R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008; 103:2007–2014. PMID: 18802997.39. Gisbert JP, Bermejo F, Pérez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009; 15:1190–1198. PMID: 19291780.
Article40. De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013; 19:2111–2117. PMID: 23883959.
Article41. Lobaton T, Rodríguez-Moranta F, Lopez A, Sánchez E, Rodríguez-Alonso L, Guardiola J. A new rapid quantitative test for fecal calprotectin predicts endoscopic activity in ulcerative colitis. Inflamm Bowel Dis. 2013; 19:1034–1042. PMID: 23470502.
Article42. Inoue K, Aomatsu T, Yoden A, Okuhira T, Kaji E, Tamai H. Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel diseases. J Gastroenterol Hepatol. 2014; 29:1406–1412. PMID: 24635100.
Article43. Nakama H, Yamamoto M, Kamijo N, et al. Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia. Hepatogastroenterology. 1999; 46:228–231. PMID: 10228797.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis
- The role of fecal calprotectin in pediatric disease
- Fecal Calprotectin in Inflammatory Bowel Disease
- Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
- Fecal Biomarkers in Inflammatory Bowel Disease