Intest Res.
2018 Oct;16(4):563-570. 10.5217/ir.2018.00020.
Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis
- Affiliations
-
- 1Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea. jskoo@korea.ac.kr
- 2Department of Psychology, Hwashin Cyber University, Busan, Korea.
- KMID: 2434158
- DOI: http://doi.org/10.5217/ir.2018.00020
Abstract
- BACKGROUND/AIMS
Ulcerative colitis undergoes periods of exacerbation and remission. Fecal calprotectin levels increase with gut inflammation and correlate with endoscopic disease activity in ulcerative colitis. Intestinal blood loss and fecal immunochemical test levels also correlate with endoscopic disease activity. This study statistically evaluated the usefulness of fecal calprotectin, fecal immunochemical test, and C-reactive protein (CRP) as markers of disease activity.
METHODS
A total 106 ulcerative colitis patients who underwent endoscopy and fecal calprotectin, fecal immunochemical test, and CRP testing, from March 2015 to August 2016, were retrospectively reviewed. Disease activity was assessed using a partial Mayo score and Mayo endoscopic score. The ability of fecal and serologic tests to reflect endoscopic disease severity was statistically evaluated.
RESULTS
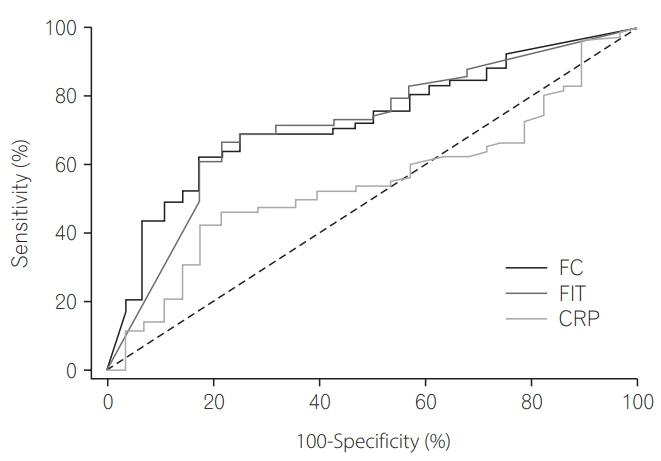
Among 106 patients, 68 underwent endoscopy and stool study within 2 weeks. In patients with mild to severe activity, fecal immunochemical test and fecal calprotectin were superior to CRP at Mayo endoscopic score detection rate. The area under the curves of fecal immunochemical test and fecal calprotectin for the detection of Mayo endoscopic score ≥1 were 0.956 and 0.942, respectively, and were superior to that of CRP (0.756). At Mayo endoscopic score, the effects of combination of fecal immunochemical test and CRP or fecal calprotectin and CRP were found to be higher than those of the independent fecal immunochemical test or fecal calprotectin.
CONCLUSIONS
Fecal immunochemical test and fecal calprotectin can effectively detect active ulcerative colitis better than remission. As these markers reflect the status of mucosal inflammation, they may reduce the requirement for invasive endoscopic examination.
MeSH Terms
Figure
Cited by 1 articles
-
Correlation of fecal calprotectin and patient-reported outcome measures in patients with ulcerative colitis
Nagesh Kamat, Sudheer K Vuyyuru, Saurabh Kedia, Pabitra Sahu, Bhaskar Kante, Peeyush Kumar, Mukesh Kumar Ranjan, Mukesh Kumar Singh, Sambuddha Kumar, Vikas Sachdev, Govind Makharia, Vineet Ahuja
Intest Res. 2022;20(2):269-273. doi: 10.5217/ir.2021.00064.
Reference
-
1. Gomes P, du Boulay C, Smith CL, Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986; 27:92–95.
Article2. Brophy MB, Nolan EM. Manganese and microbial pathogenesis: sequestration by the mammalian immune system and utilization by microorganisms. ACS Chem Biol. 2015; 10:641–651.
Article3. Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008; 103:162–169.
Article4. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.
Article5. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000; 119:15–22.
Article6. Levi Z, Rozen P, Hazazi R, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007; 146:244–255.
Article7. Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013; 108:83–89.
Article8. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article9. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983; 148:839–843.
Article10. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845.
Article11. Vilkin A, Rozen P, Levi Z, et al. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol. 2005; 100:2519–2525.
Article12. Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces: a methodologic study. Scand J Gastroenterol. 1992; 27:793–798.
Article13. Rose IS, Young GP, St John DJ, Deacon MC, Blake D, Henderson RW. Effect of ingestion of hemoproteins on fecal excretion of hemes and porphyrins. Clin Chem. 1989; 35:2290–2296.
Article14. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.
Article15. Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004; 53:1813–1816.
Article16. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008; 14:40–46.
Article17. Kato S, Ishibashi A, Kani K, Yakabi K. Optimized management of ulcerative proctitis: when and how to use mesalazine suppository. Digestion. 2018; 97:59–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Immunochemical Test and Fecal Calprotectin Measurement Are Noninvasive Monitoring Tools for Predicting Endoscopic Activity in Patients with Ulcerative Colitis
- Fecal Immunochemical Test and Fecal Calprotectin Results Show Different Profiles in Disease Monitoring for Ulcerative Colitis
- Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin?
- Beyond mucosal healing: fecal calprotectin and the path toward histologic remission in ulcerative colitis
- Fecal Calprotectin in Inflammatory Bowel Disease