Korean J Physiol Pharmacol.
2019 Jan;23(1):81-87. 10.4196/kjpp.2019.23.1.81.
Chronic administration of ketamine ameliorates the anxiety- and aggressive-like behavior in adolescent mice induced by neonatal maternal separation
- Affiliations
-
- 1Department of Physiology and Biophysics, School of Medicine, Eulji University, Daejeon 34824, Korea. hans0424@eulji.ac.kr, ssmin@eulji.ac.kr
- KMID: 2443619
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.81
Abstract
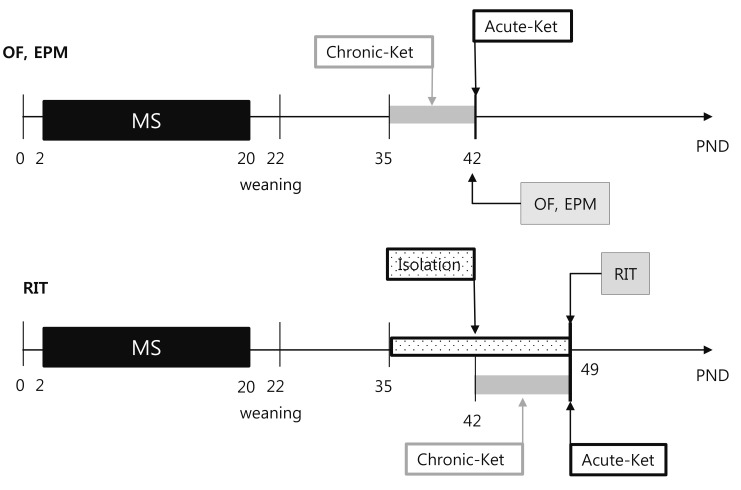
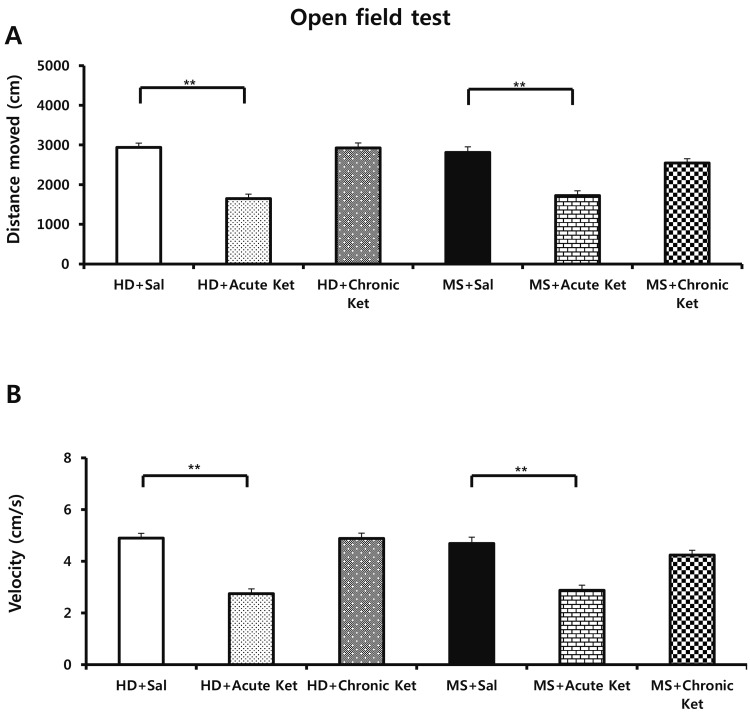
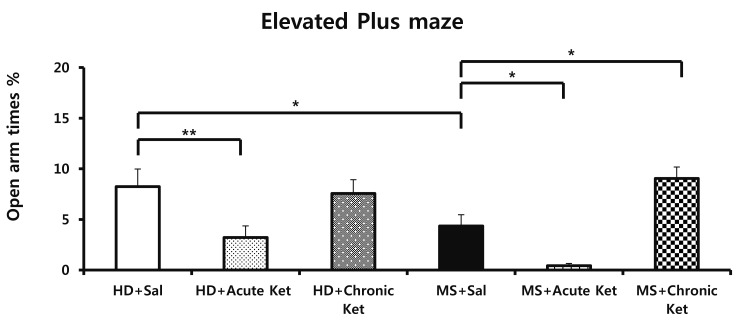
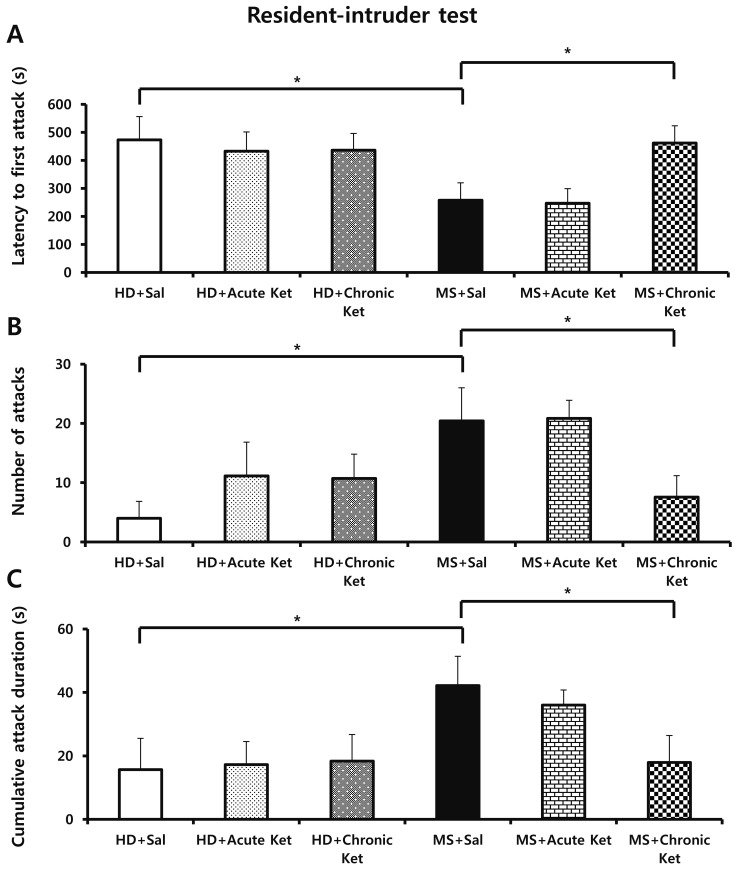
- Ketamine has long been used as an anesthetic agent. However, ketamine use is associated with numerous side effects, including flashbacks, amnesia, delirium, and aggressive or violent behavior. Ketamine has also been abused as a cocktail with ecstasy, cocaine, and methamphetamine. Several studies have investigated therapeutic applications of ketamine, demonstrating its antidepressant and anxiolytic effects in both humans and rodents. We recently reported that neonatal maternal separation causes enhanced anxiety- and aggressive-like behaviors in adolescent. In the present study, we evaluated how acute and chronic ketamine administration affected the behavioral consequences of neonatal maternal separation in adolescent mice. Litters were separated from dams for 4 hours per day for 19 days beginning after weaning. Upon reaching adolescence (post-natal day 35-49), mice were acutely (single injection) or chronically (7 daily injections) treated with a sub-anesthetic dose (15 mg/kg) of ketamine. At least 1 h after administration of ketamine, mice were subjected to open-field, elevated-plus maze, and resident-intruder tests. We found that acute ketamine treatment reduced locomotor activity. In contrast, chronic ketamine treatment decreased anxiety, as evidenced by increased time spent on open arms in the elevated-plus maze, and remarkably reduced the number and duration of attacks. In conclusion, the present study suggests that ketamine has potential for the treatment of anxiety and aggressive or violent behaviors.
Keyword
MeSH Terms
Figure
Reference
-
1. David-Ferdon C, Simon TR, Spivak H, Gorman-Smith D, Savannah SB, Listenbee RL, Iskander J. Centers for Disease C, Prevention. CDC grand rounds: preventing youth violence. MMWR Morb Mortal Wkly Rep. 2015; 64:171–174. PMID: 25719677.2. Maeng S, Zarate CA Jr. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007; 9:467–474. PMID: 18221626.
Article3. Michelotti P, Quadros VA, Pereira ME, Rosemberg DB. Ketamine modulates aggressive behavior in adult zebrafish. Neurosci Lett. 2018; 684:164–168. PMID: 30102959.
Article4. Hirota K, Lambert DG. Ketamine: its mechanism(s) of action and unusual clinical uses. Br J Anaesth. 1996; 77:441–444. PMID: 8942324.
Article5. Bolshakov KV, Gmiro VE, Tikhonov DB, Magazanik LG. Determinants of trapping block of N-methyl-d-aspartate receptor channels. J Neurochem. 2003; 87:56–65. PMID: 12969252.
Article6. Brown JA, Ramikie TS, Schmidt MJ, Báldi R, Garbett K, Everheart MG, Warren LE, Gellért L, Horváth S, Patel S, Mirnics K. Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol Psychiatry. 2015; 20:1499–1507. PMID: 25623945.
Article7. Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007; 318:1645–1647. PMID: 18063801.
Article8. MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987; 58:251–266. PMID: 2443623.
Article9. Shin SY, Han SH, Woo RS, Jang SH, Min SS. Adolescent mice show anxiety- and aggressive-like behavior and the reduction of long-term potentiation in mossy fiber-CA3 synapses after neonatal maternal separation. Neuroscience. 2016; 316:221–231. PMID: 26733385.
Article10. Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann N Y Acad Sci. 1999; 877:756–759. PMID: 10415699.
Article11. Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000; 12:5–12. PMID: 10692138.
Article12. Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004; 29:1988–1993. PMID: 15316569.
Article13. Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012; 72:499–504. PMID: 22552045.
Article14. Chen YW, Sherpa AD, Aoki C. Single injection of ketamine during mid-adolescence promotes long-lasting resilience to activity-based anorexia of female mice by increasing food intake and attenuating hyperactivity as well as anxiety-like behavior. Int J Eat Disord. 2018; 51:1020–1025. PMID: 30102796.
Article15. Nagy LR, Featherstone RE, Hahn CG, Siegel SJ. Delayed emergence of behavioral and electrophysiological effects following juvenile ketamine exposure in mice. Transl Psychiatry. 2015; 5:e635. PMID: 26371763.
Article16. Li M, Xue X, Shao S, Shao F, Wang W. Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res. 2013; 1518:82–90. PMID: 23623774.
Article17. Harris T, Brown GW, Bifulco A. Loss of parent in childhood and adult psychiatric disorder: the role of lack of adequate parental care. Psychol Med. 1986; 16:641–659. PMID: 3763778.
Article18. Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998; 16:149–164. PMID: 9785112.
Article19. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982; 56:119–136. PMID: 6892475.
Article20. Takahashi RN, Morato GS, Monteiro-de-Lima TC. Effects of ketamine on experimental animal models of aggression. Braz J Med Biol Res. 1984; 17:171–178. PMID: 6542807.21. McEwen BS, Albeck D, Cameron H, Chao HM, Gould E, Hastings N, Kuroda Y, Luine V, Magariños AM, McKittrick CR, Orchinik M, Pavlides C, Vaher P, Watanabe Y, Weiland N. Stress and the brain: a paradoxical role for adrenal steroids. Vitam Horm. 1995; 51:371–402. PMID: 7483328.
Article22. Clarke M, Razmjou S, Prowse N, Dwyer Z, Litteljohn D, Pentz R, Anisman H, Hayley S. Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology. 2017; 112:210–220. PMID: 27106168.
Article23. Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci. 2010; 4:12. DOI: 10.3389/fnbeh.2010.00012. PMID: 20407578.
Article24. Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997; 94:14002–14008. PMID: 9391142.25. Baudry M, Oliver M, Creager R, Wieraszko A, Lynch G. Increase in glutamate receptors following repetitive electrical stimulation in hippocampal slices. Life Sci. 1980; 27:325–330. PMID: 7412479.
Article26. Covington HE 3rd, Newman EL, Tran S, Walton L, Hayek W, Leonard MZ, DeBold JF, Miczek KA. The urge to fight: persistent escalation by alcohol and role of NMDA receptors in mice. Front Behav Neurosci. 2018; 12:206. DOI: 10.3389/fnbeh.2018.00206. PMID: 30271332.
Article27. Crawford TN, Cohen PR, Chen H, Anglin DM, Ehrensaft M. Early maternal separation and the trajectory of borderline personality disorder symptoms. Dev Psychopathol. 2009; 21:1013–1030. PMID: 19583895.
Article28. Haller J, Harold G, Sandi C, Neumann ID. Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J Neuroendocrinol. 2014; 26:724–738. PMID: 25059307.
Article29. Kendall T, Pilling S, Tyrer P, Duggan C, Burbeck R, Meader N, Taylor C. Guideline Development Groups. Borderline and antisocial personality disorders: summary of NICE guidance. BMJ. 2009; 338:b93. DOI: 10.1136/bmj.693. PMID: 19176682.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect Neonatal Ketamine Treatment on Exploratory and Anxiety-like Behaviours in Adulthood
- Maternal Separation Does Not Produce a Significant Behavioral Change in Mice
- Antidepressant Effects of Ketamine on Depression-like Behavior in Juvenile Mice after Neonatal Dexamethasone Exposure
- Maternal separation in mice leads to anxiety-like/aggressive behavior and increases immunoreactivity for glutamic acid decarboxylase and parvalbumin in the adolescence ventral hippocampus
- Dissociated Antidepressant and Analgesic Effects of Intravenous Ketamine in Patients with Chronic Pain