Korean J Physiol Pharmacol.
2019 Jan;23(1):21-28. 10.4196/kjpp.2019.23.1.21.
Swertiamarin ameliorates carbon tetrachloride-induced hepatic apoptosis via blocking the PI3K/Akt pathway in rats
- Affiliations
-
- 1Department of Pharmacy, General Hospital of the Yangtze River Shipping, Wuhan 430022, China.
- 2Department of Pharmacy, Huanggang Central Hospital, Huanggang 438000, China.
- 3Department of Pharmacy, Wuhan NO.4 Hospital, Wuhan Puai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. goodgravity@foxmail.com, hydrinsk@sina.com
- KMID: 2443612
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.21
Abstract
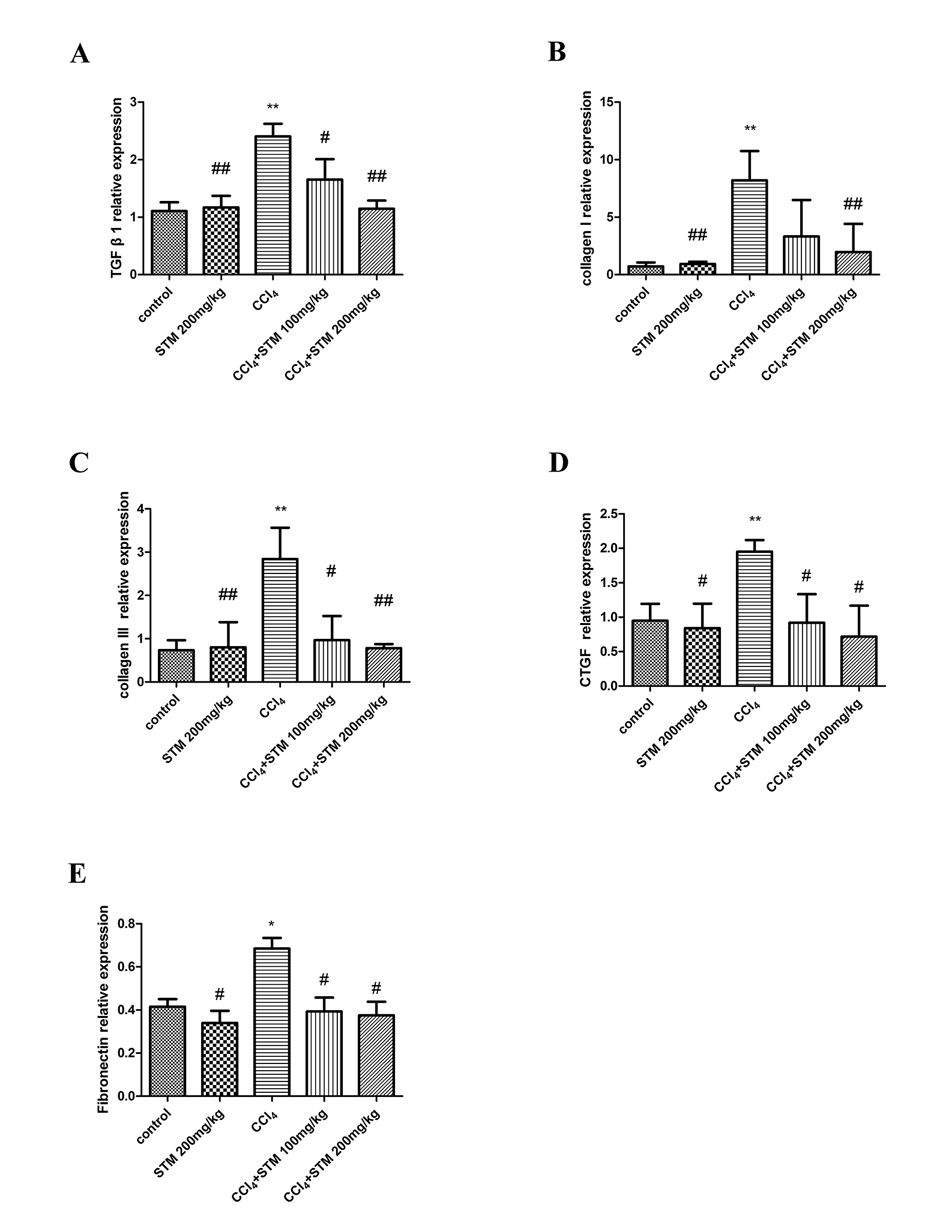
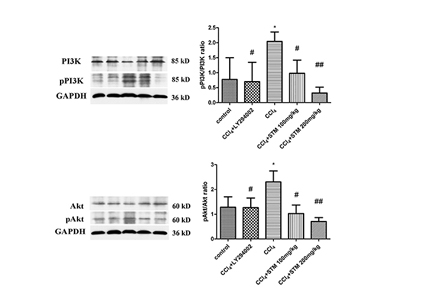
- Swertiamarin (STM) is an iridoid compound that is present in the Gentianaceae swertia genus. Here we investigated antiapoptotic effects of STM on carbon tetrachloride (CCl₄)-induced liver injury and its possible mechanisms. Adult male Sprague Dawley rats were randomly divided into a control group, an STM 200 mg/kg group, a CCl₄ group, a CCl₄+STM 100 mg/kg group, and a CCl₄+STM 200 mg/kg group. Rats in experimental groups were subcutaneously injected with 40% CCl₄ twice weekly for 8 weeks. STM (100 and 200 mg/kg per day) was orally given to experimental rats by gavage for 8 consecutive weeks. Hepatocyte apoptosis was determined by TUNEL assay and the expression levels of Bcl-2, Bax, and cleaved caspase-3 proteins were evaluated by western blot analysis. The expression of TGF-β1, collagen I, collagen III, CTGF and fibronectin mRNA were estimated by qRT-PCR. The results showed that STM significantly reduced the number of TUNEL-positive cells compared with the CCl₄ group. The levels of Bax and cleaved caspase-3 proteins, and TGF-β1, collagen I, collagen III, CTGF, and fibronectin mRNA were significantly reduced by STM compared with the CCl₄ group. In addition, STM markedly abrogated the repression of Bcl-2 by CCl₄. STM also attenuated the activation of the PI3K/Akt pathway in the liver. These results suggested that STM ameliorated CCl₄-induced hepatocyte apoptosis in rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Delire B, Stärkel P, Leclercq I. Animal models for fibrotic liver diseases: What we have, what we need, and what is under development. J Clin Transl Hepatol. 2015; 3:53–66.2. Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012; 7:105–117.
Article3. Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap Adv Gastroenterol. 2011; 4:391–417.
Article4. Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001; 21:311–335.
Article5. Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002; 36:200–209.
Article6. Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 2012; 12:26.
Article7. Liang B, Guo XL, Jin J, Ma YC, Feng ZQ. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J Gastroenterol. 2015; 21:5271–5280.
Article8. Lee TY, Chang HH, Wu MY, Lin HC. Yin-Chen-Hao-Tang ameliorates obstruction-induced hepatic apoptosis in rats. J Pharm Pharmacol. 2007; 59:583–590.
Article9. Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002; 192:131–137.
Article10. Zhang J, Dong M, Li L, Fan Y, Pathre P, Dong J, Lou D, Wells JM, Olivares-Villagómez D, Van Kaer L, Wang X, Xu M. Endonuclease G is required for early embryogenesis and normal apoptosis in mice. Proc Natl Acad Sci U S A. 2003; 100:15782–15787.
Article11. Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta. 1998; 1366:151–165.
Article12. Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997; 91:479–489.
Article13. Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 2004; 557:14–20.
Article14. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003; 33:105–136.
Article15. Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Z Gastroenterol. 2007; 45:43–50.
Article16. Jaishree V, Badami S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J Ethnopharmacol. 2010; 130:103–106.
Article17. Wu T, Li J, Li Y, Song H. Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol Biochem. 2017; 41:2242–2254.
Article18. Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015; 64:830–841.
Article19. Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int. 2006; 26:8–22.20. Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005; 45:605–628.
Article21. Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003; 38:Suppl 1. S38–S53.
Article22. Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001; 21:351–372.
Article23. Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979; 76:710–719.
Article24. Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990; 85:1833–1843.
Article25. Gressner OA, Lahme B, Demirci I, Gressner AM, Weiskirchen R. Differential effects of TGF-beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007; 47:699–710.26. Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008; 28:1065–1079.
Article27. Liu XY, Liu RX, Hou F, Cui LJ, Li CY, Chi C, Yi E, Wen Y, Yin CH. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol Med Rep. 2016; 14:3669–3675.
Article28. Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, Sun M, Liu C, Liu P. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complement Altern Med. 2012; 12:51.
Article29. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008; 134:1655–1669.
Article30. Lee TY, Chang HH, Wang GJ, Chiu JH, Yang YY, Lin HC. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J Pharm Pharmacol. 2006; 58:659–665.
Article31. Ding WX, Nam Ong C. Role of oxidative stress and mitochondrial changes in cyanobacteria-induced apoptosis and hepatotoxicity. FEMS Microbiol Lett. 2003; 220:1–7.
Article32. Tien YC, Liao JC, Chiu CS, Huang TH, Huang CY, Chang WT, Peng WH. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int J Mol Sci. 2011; 12:4053–4067.
Article33. Sun F, Hamagawa E, Tsutsui C, Ono Y, Ogiri Y, Kojo S. Evaluation of oxidative stress during apoptosis and necrosis caused by carbon tetrachloride in rat liver. Biochim Biophys Acta. 2001; 1535:186–191.
Article34. Brown GC, Borutaite V. Nitric oxide, cytochrome c and mitochondria. Biochem Soc Symp. 1999; 66:17–25.
Article35. Son MK, Ryu YL, Jung KH, Lee H, Lee HS, Yan HH, Park HJ, Ryu JK, Suh JK, Hong S, Hong SS. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci Rep. 2013; 3:3470.
Article36. Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009; 50:1512–1523.
Article37. Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, Rajaraman S, Evers BM. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008; 294:G1401–G1410.
Article38. Wang Q, Wen R, Lin Q, Wang N, Lu P, Zhu X. Wogonoside shows antifibrotic effects in an experimental regression model of hepatic fibrosis. Dig Dis Sci. 2015; 60:3329–3339.
Article39. Chen X, Bian M, Zhang C, Kai J, Yao Z, Jin H, Lu C, Shao J, Chen A, Zhang F, Zheng S. Dihydroartemisinin inhibits ER stress-mediated mitochondrial pathway to attenuate hepatocyte lipoapoptosis via blocking the activation of the PI3K/Akt pathway. Biomed Pharmacother. 2018; 97:975–984.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphologic Change of Rat Liver Induced by Repeated Administration of Carbon Tetrachloride and Dimethylnitrosamine
- Carbon tetrachloride (CCl4)-induced hepatic fibrosis in the rat
- Developmental Exposure to Di-(2-ethylhexyl) Phthalate Induces Cerebellar Granule Cell Apoptosis via the PI3K/AKT Signaling Pathway
- Curcumin targets vascular endothelial growth factor viaactivating the PI3K/Akt signaling pathway and improves brainhypoxic-ischemic injury in neonatal rats
- Hypothalamic-Pituitary-Gonadal Function in Hepatic Failure, Experimental Study