World J Mens Health.
2019 May;37(2):240-248. 10.5534/wjmh.180082.
Persistent Erectile Dysfunction after Discontinuation of 5-Alpha Reductase Inhibitor Therapy in Rats Depending on the Duration of Treatment
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. drswlee@skku.edu
- 2Department of Physiology and Biophysics, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Urology, Chonbuk National University College of Medicine and Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute and Clinical Trial Center of Medical Device of Chonbuk National University, Jeonju, Korea.
- KMID: 2443240
- DOI: http://doi.org/10.5534/wjmh.180082
Abstract
- PURPOSE
The current study is aimed to assess whether a longer duration of 5α-reductase inhibitor (5α-RI) exposure was associated with higher rate of permanent erectile dysfunction (ED) in a rat model.
MATERIALS AND METHODS
Male Sprague-Dawley rats (n=76) were assigned to five groups: (i) normal control group; (ii) dutasteride (0.5 mg/rat/d) for 4-weeks group; (iii) dutasteride for 4-weeks plus 2-weeks of resting group; (iv) dutasteride for 8-weeks group; and (v) dutasteride for 8-weeks plus 2-weeks of resting group. In vivo erectile responses to electrical stimulation, and changes of fibrotic factors and smooth muscle/collagen contents in the corpus cavernosum were evaluated in each group.
RESULTS
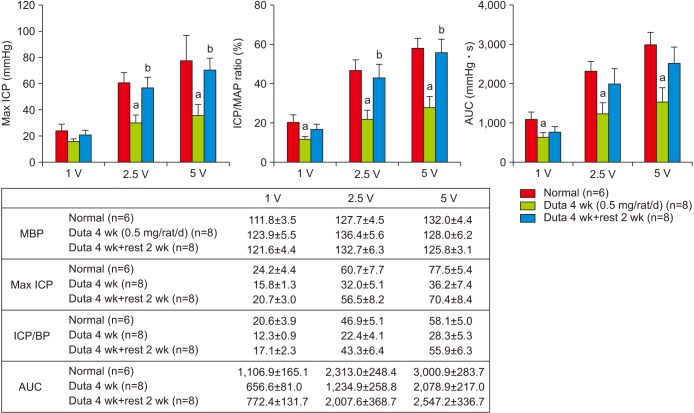
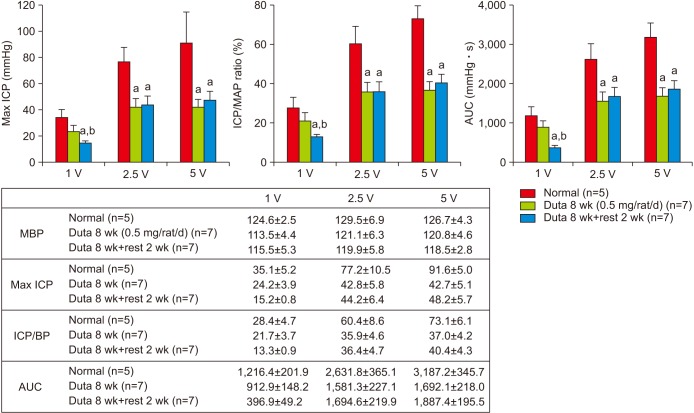
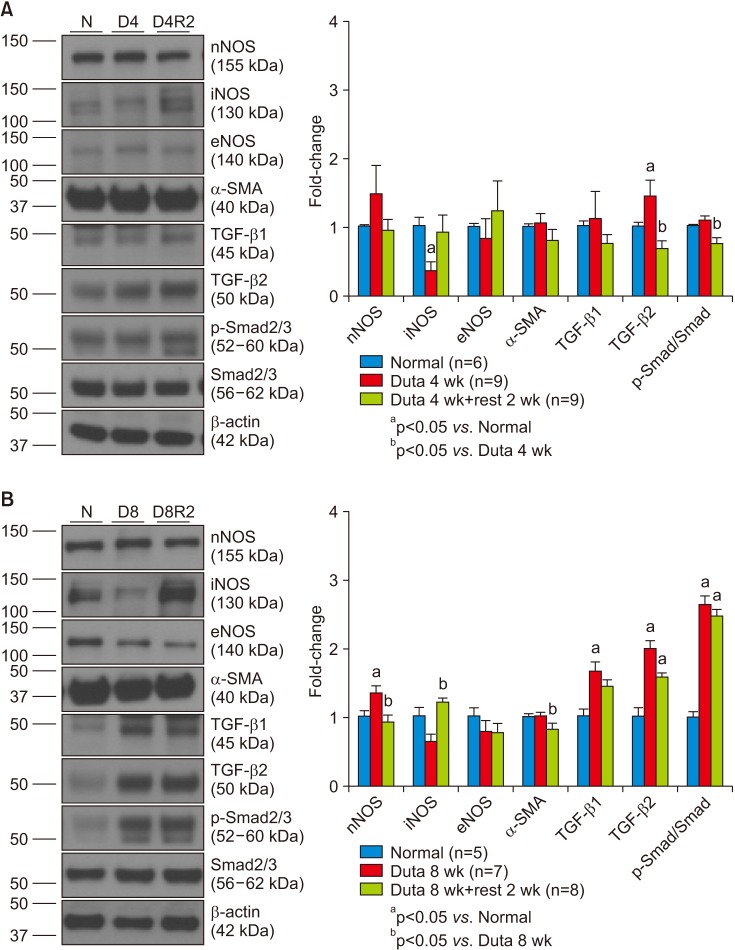
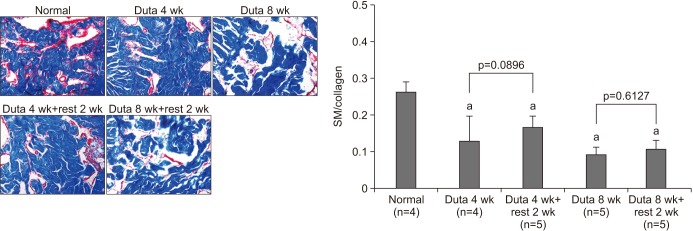
Dutasteride administration for 4 and 8 weeks significantly decreased erectile parameters compared with the control group. Reduced erectile responses were recovered during 2 weeks of drug-free time in the 4-week treatment group, but were not in the 8-week group. Protein levels of fibrosis-related factors transforming growth factor (TGF)-β1, TGF-β2, and p-Smad/Smad (Smad 2/3) in the corpus cavernosum showed no significant change after 4 weeks of dutasteride oral administration, but were enhanced after 8 weeks. Dutasteride markedly decreased smooth muscle content and increased collagen after 4 and 8 weeks of use, but no nuclear size changes; however, neither group showed significant improvement in the smooth muscle to collagen ratio after the rest period.
CONCLUSIONS
Our study showed that recovery from ED depended on the duration of medication, and administration of dutasteride for more than 8-weeks in rats could result in irreversible ED even after discontinuation of medication.
MeSH Terms
-
5-alpha Reductase Inhibitors
Administration, Oral
Animals
Collagen
Dutasteride
Electric Stimulation
Erectile Dysfunction*
Finasteride
Humans
Male
Models, Animal
Muscle, Smooth
Oxidoreductases*
Rats*
Rats, Sprague-Dawley
Transforming Growth Factors
5-alpha Reductase Inhibitors
Collagen
Dutasteride
Finasteride
Oxidoreductases
Transforming Growth Factors
Figure
Reference
-
1. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984; 132:474–479. PMID: 6206240.
Article2. McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011; 185:1793–1803. PMID: 21420124.
Article3. Filson CP, Hollingsworth JM, Clemens JQ, Wei JT. The efficacy and safety of combined therapy with α-blockers and anticholinergics for men with benign prostatic hyperplasia: a meta-analysis. J Urol. 2013; 190:2153–2160. PMID: 23727412.
Article4. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–224. PMID: 12824459.
Article5. Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011; 8:1747–1753. PMID: 21418145.
Article6. Irwig MS. Persistent sexual and nonsexual adverse effects of finasteride in younger men. Sex Med Rev. 2014; 2:24–35. PMID: 27784541.
Article7. Ganzer CA, Jacobs AR, Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am J Mens Health. 2015; 9:222–228. PMID: 24928450.8. Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, et al. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J Sex Med. 2013; 10:2598–2603. PMID: 23890183.
Article9. Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry. 2012; 73:1220–1223. PMID: 22939118.
Article10. Pinsky MR, Gur S, Tracey AJ, Harbin A, Hellstrom WJ. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med. 2011; 8:3066–3074. PMID: 21834872.
Article11. Zhang MG, Wu W, Zhang CM, Wang XJ, Gao PJ, Lu YL, et al. Effects of oral finasteride on erectile function in a rat model. J Sex Med. 2012; 9:1328–1336. PMID: 22375859.
Article12. Oztekin CV, Gur S, Abdulkadir NA, Lokman U, Akdemir AÖ, Cetinkaya M, et al. Incomplete recovery of erectile function in rat after discontinuation of dual 5-alpha reductase inhibitor therapy. J Sex Med. 2012; 9:1773–1781. PMID: 22568670.13. Kiguradze T, Temps WH, Yarnold PR, Cashy J, Brannigan RE, Nardone B, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017; 5:e3020. PMID: 28289563.
Article14. Traish AM, Haider KS, Doros G, Haider A. Finasteride, not tamsulosin, increases severity of erectile dysfunction and decreases testosterone levels in men with benign prostatic hyperplasia. Horm Mol Biol Clin Investig. 2015; 23:85–96.
Article15. Sung HH, Kang SJ, Chae MR, Kim HK, Park JK, Kim CY, et al. Effect of BKCa channel opener LDD175 on erectile function in an in vivo diabetic rat model. J Sex Med. 2017; 14:59–68. PMID: 27989487.16. Gisleskog PO, Hermann D, Hammarlund-Udenaes M, Karlsson MO. The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. Br J Clin Pharmacol. 1999; 47:53–58. PMID: 10073740.
Article17. Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology. 2009; 73:802–806. PMID: 19193422.18. Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men. J Urol. 2008; 179:2333–2338. PMID: 18423697.19. Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005; 21:775–777. PMID: 15925305.
Article20. Bramson HN, Hermann D, Batchelor KW, Lee FW, James MK, Frye SV. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997; 282:1496–1502. PMID: 9316864.21. Dey P. Cancer nucleus: morphology and beyond. Diagn Cytopathol. 2010; 38:382–390. PMID: 19894267.
Article22. Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999; 140:1861–1868. PMID: 10098525.23. Chung CC, Kao YH, Chen YJ, Chen YJ. Androgen modulates cardiac fibrosis contributing to gender differences on heart failure. Aging Male. 2013; 16:22–27. PMID: 23356882.
Article24. Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007; 74:196–206. PMID: 17376414.25. Crook JM, O'Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012; 367:895–903. PMID: 22931259.
Article26. Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TL. FinnProstate Group. Advanced prostate cancer treated with intermittent or continuous androgen deprivation in the randomised FinnProstate study VII: quality of life and adverse effects. Eur Urol. 2013; 63:111–120. PMID: 22857983.
Article27. Schulman C, Cornel E, Matveev V, Tammela TL, Schraml J, Bensadoun H, et al. Intermittent versus continuous androgen deprivation therapy in patients with relapsing or locally advanced prostate cancer: a phase 3b randomised study (ICELAND). Eur Urol. 2016; 69:720–727. PMID: 26520703.
Article28. Corona G, Tirabassi G, Santi D, Maseroli E, Gacci M, Dicuio M, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017; 5:671–678. PMID: 28453908.
Article29. Cindolo L, Pirozzi L, Fanizza C, Romero M, Tubaro A, Autorino R, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015; 68:418–425. PMID: 25465970.
Article30. Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010; 57:123–131. PMID: 19825505.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Chronic Treatment with a Type 5 Phosphodiesterase Inhibitor on Erectile Function in Diabetic Rats
- Factors Influencing Continued Usage of Intracavernosal Injections for Erectile Dysfunction: A Retrospective Analysis
- Patient's Factors Correlated with Prostate Volume Recovery after 5 Alpha Reductase Inhibitor Discontinuation
- Time-dependent Changes in Erectile Function and Responsiveness to Phosphodiesterase Type 5 Inhibitor in Streptozotocin-induced Diabetic Rats
- Benign Prostatic Hyperplasia and Sexual Dysfunction