Dement Neurocogn Disord.
2016 Sep;15(3):88-91. 10.12779/dnd.2016.15.3.88.
Human Herpes Virus 6 Encephalitis Following Bone Marrow Transplantation with Uncommon Magnetic Resonance Imaging Findings
- Affiliations
-
- 1Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jhlee@amc.seoul.kr
- KMID: 2442851
- DOI: http://doi.org/10.12779/dnd.2016.15.3.88
Abstract
- BACKGROUND
Human Herpes Virus 6 (HHV6) is commonly associated with encephalitis following bone marrow transplantation. However, hippocampal atrophy and global hypometabolism are rare findings in HHV6 encephalitis.
CASE REPORT
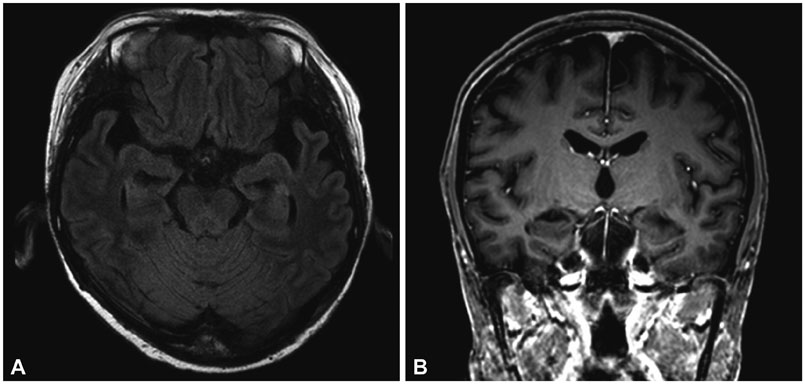
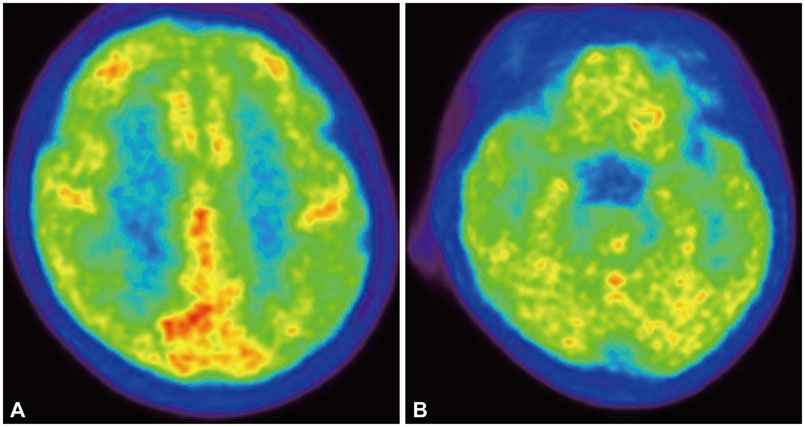
A 41-year-old right-handed woman with acute lymphoblastic leukemia presented with fever and mental changes 2 weeks after receiving a sibling bone marrow transplant. The patient's cerebrospinal fluid (CSF) was positive for HHV-6 deoxyribonucleic acid (DNA), but was negative for other viral DNA. Brain magnetic resonance imaging revealed atrophic changes in bilateral medial temporal lobes. Following 4 weeks of ganciclovir therapy, a CSF exam was negative for HHV-6 DNA and the patient's neurological symptoms partially improved. However, she was disoriented and had severe retrograde and anterograde amnesia. 18F-fluorodeoxyglucose-positron emission tomography indicated global hypometabolism in the medial temporal lobes and the fronto-parietal cortices.
CONCLUSIONS
This is a rare and unusual case of hippocampal atrophy in the acute stage of HHV6 encephalitis. Our imaging findings may reflect the chronic indolent course of HHV6 encephalitis.
Keyword
MeSH Terms
-
Adult
Amnesia, Anterograde
Amnesia, Retrograde
Atrophy
Bone Marrow Transplantation*
Bone Marrow*
Brain
Cerebrospinal Fluid
DNA
DNA, Viral
Encephalitis*
Female
Fever
Ganciclovir
Herpesvirus 6, Human
Humans*
Limbic Encephalitis
Magnetic Resonance Imaging*
Precursor Cell Lymphoblastic Leukemia-Lymphoma
Siblings
Temporal Lobe
DNA
DNA, Viral
Ganciclovir
Figure
Reference
-
1. Yoshikawa T. Human herpesvirus 6 infection in hematopoietic stem cell transplant patients. Br J Haematol. 2004; 124:421–432.
Article2. Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace M, Armstrong RW, et al. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. 2005; 40:890–893.
Article3. Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007; 69:156–165.
Article4. Vu T, Carrum G, Hutton G, Heslop HE, Brenner MK, Kamble R. Human herpesvirus-6 encephalitis following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007; 39:705–709.
Article5. Drobyski WR, Knox KK, Majewski D, Carrigan DR. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994; 330:1356–1360.
Article6. Gorniak RJ, Young GS, Wiese DE, Marty FM, Schwartz RB. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. AJNR Am J Neuroradiol. 2006; 27:887–891.
Article7. Noguchi T, Mihara F, Yoshiura T, Togao O, Atsumi K, Matsuura T, et al. MR imaging of human herpesvirus-6 encephalopathy after hematopoietic stem cell transplantation in adults. AJNR Am J Neuroradiol. 2006; 27:2191–2195.
Article8. Hubele F, Bilger K, Kremer S, Imperiale A, Lioure B, Namer IJ. Sequential FDG PET and MRI findings in a case of human herpes virus 6 limbic encephalitis. Clin Nucl Med. 2012; 37:716–717.
Article9. Ogata M, Fukuda T, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant. 2015; 50:1030–1036.
Article10. Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013; 57:671–681.
Article11. Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006; 193:68–79.
Article12. Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986; 234:596–601.
Article13. Dominguez G, Dambaugh TR, Stamey FR, Dewhurst S, Inoue N, Pellett PE. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J Virol. 1999; 73:8040–8052.
Article14. Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988; 1:1065–1067.15. Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005; 352:768–776.16. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005; 18:217–245.
Article17. Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001; 50:612–619.
Article18. Provenzale JM, van Landingham K, White LE. Clinical and imaging findings suggesting human herpesvirus 6 encephalitis. Pediatr Neurol. 2010; 42:32–39.
Article19. Milner B, Klein D. Loss of recent memory after bilateral hippocampal lesions: memory and memories-looking back and looking forward. J Neurol Neurosurg Psychiatry. 2016; 87:230.
Article20. Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000; 12:103–113.
Article21. Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20:11–21.
Article22. Yamane A, Mori T, Suzuki S, Mihara A, Yamazaki R, Aisa Y, et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transplant. 2007; 13:100–106.
Article23. Millichap JJ, Millichap JG. Role of HHV-6B Infection in Mesial Temporal Lobe Epilepsy. Pediatr Neurol Briefs. 2015; 29:40.
Article24. Kawamura Y, Nakayama A, Kato T, Miura H, Ishihara N, Ihira M, et al. Pathogenic role of human herpesvirus 6B infection in mesial temporal lobe epilepsy. J Infect Dis. 2015; 212:1014–1021.
Article25. Leibovitch EC, Jacobson S. Human herpesvirus 6 as a viral trigger in mesial temporal lobe epilepsy. J Infect Dis. 2015; 212:1011–1013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Encephalitis by Co-infection with A/H1N1 Influenza and Herpes Simplex Virus in an Adult Patient
- Serial MRI, SPECT and FDG-PET Findings in a Case of Herpes Simplex Encephalitis
- Ramsay Hunt Syndrome Complicated by Meningoencephalitis and Radiologic findings: a Rare Case Report
- A Case of Neurosyphilis Radiologically Mimicking Herpes Simplex Encephalitis
- A Case of Limbic Encephalitis Developed after Allogeneic Stem Cell Transplantation