Korean J Radiol.
2019 May;20(5):801-811. 10.3348/kjr.2018.0453.
Monitoring Response to Neoadjuvant Chemotherapy of Primary Osteosarcoma Using Diffusion Kurtosis Magnetic Resonance Imaging: Initial Findings
- Affiliations
-
- 1Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China. yaoweiwuhuan@163.com
- 2Department of Pathology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China.
- 3Department of Orthopedics, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China.
- KMID: 2442714
- DOI: http://doi.org/10.3348/kjr.2018.0453
Abstract
OBJECTIVE
To determine whether diffusion kurtosis imaging (DKI) is effective in monitoring tumor response to neoadjuvant chemotherapy in patients with osteosarcoma.
MATERIALS AND METHODS
Twenty-nine osteosarcoma patients (20 men and 9 women; mean age, 17.6 ± 7.8 years) who had undergone magnetic resonance imaging (MRI) and DKI before and after neoadjuvant chemotherapy were included. Tumor volume, apparent diffusion coefficient (ADC), mean diffusivity (MD), mean kurtosis (MK), and change ratio (ΔX) between pre- and post-treatment were calculated. Based on histologic response, the patients were divided into those with good response (≥ 90% necrosis, n = 12) and those with poor response (< 90% necrosis, n = 17). Several MRI parameters between the groups were compared using Student's t test. The correlation between image indexes and tumor necrosis was determined using Pearson's correlation, and diagnostic performance was compared using receiver operating characteristic curves.
RESULTS
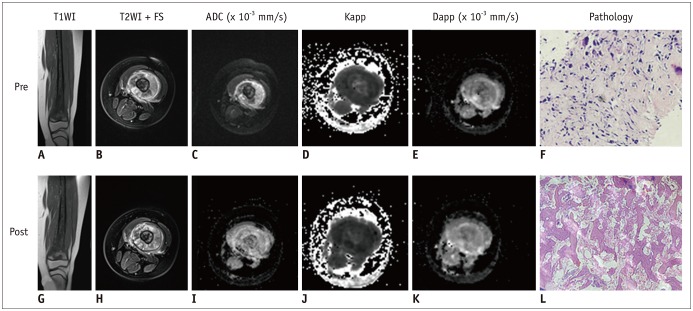
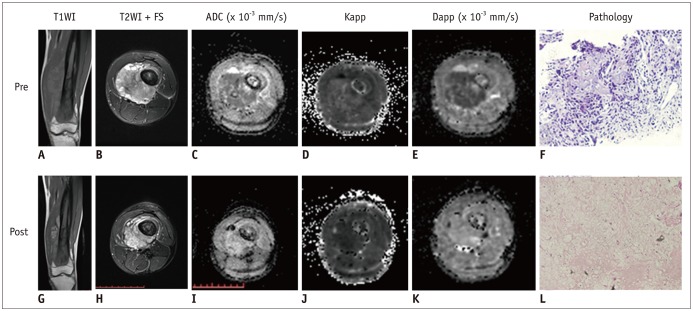
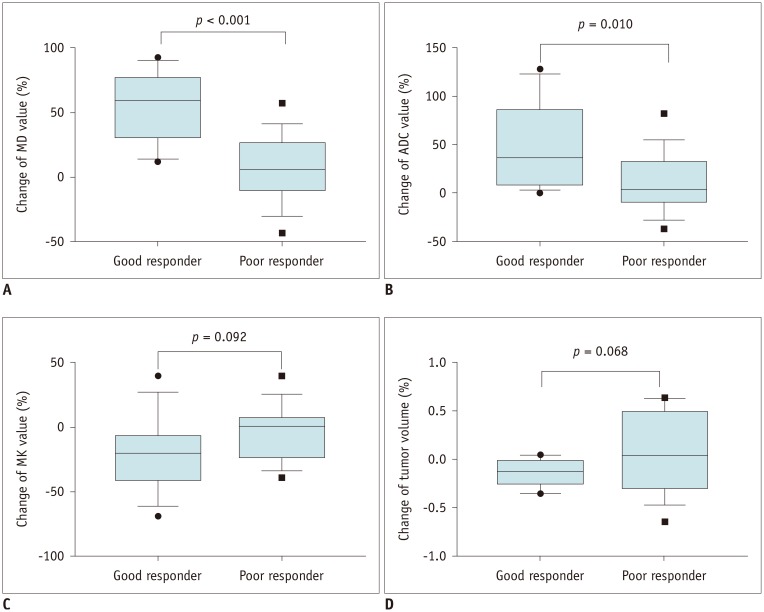
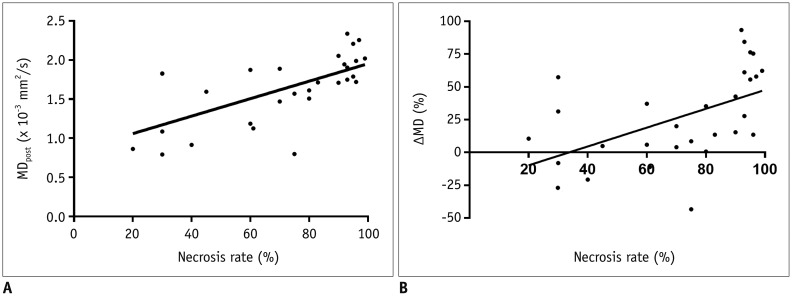
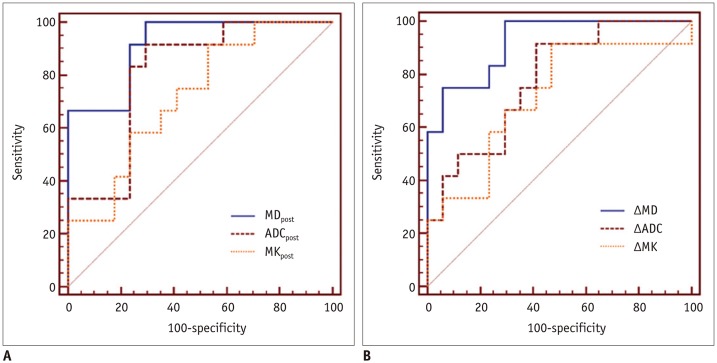
In good responders, MDpost, ADCpost, and MKpost values were significantly higher than in poor responders (p < 0.001, p < 0.001, and p = 0.042, respectively). The ΔMD and ΔADC were also significantly higher in good responders than in poor responders (p < 0.001 and p = 0.01, respectively). However, no significant difference was observed in ΔMK (p = 0.092). MDpost and ΔMD showed high correlations with tumor necrosis rate (r = 0.669 and r = 0.622, respectively), and MDpost had higher diagnostic performance than ADCpost (p = 0.037) and MKpost (p = 0.011). Similarly, ΔMD also showed higher diagnostic performance than ΔADC (p = 0.033) and ΔMK (p = 0.037).
CONCLUSION
MD is a promising biomarker for monitoring tumor response to preoperative chemotherapy in patients with osteosarcoma.
Keyword
MeSH Terms
Figure
Reference
-
1. de Baere T, Vanel D, Shapeero LG, Charpentier A, Terrier P, di Paola M. Osteosarcoma after chemotherapy: evaluation with contrast material-enhanced subtraction MR imaging. Radiology. 1992; 185:587–592. PMID: 1410378.
Article2. Bielack SS, Hecker-Nolting S, Blattmann C, Kager L. Advances in the management of osteosarcoma. F1000Res. 2016; 5:2767. PMID: 27990273.
Article3. Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994; 12:423–431. PMID: 8113851.
Article4. Denecke T, Hundsdorfer P, Misch D, Steffen IG, Schonberger S, Furth C, et al. Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging. 2010; 37:1842–1853. PMID: 20505933.
Article5. Kubo T, Furuta T, Johan MP, Adachi N, Ochi M. Percent slope analysis of dynamic magnetic resonance imaging for assessment of chemotherapy response of osteosarcoma or Ewing sarcoma: systematic review and meta-analysis. Skeletal Radiol. 2016; 45:1235–1242. PMID: 27229874.
Article6. Brisse H, Ollivier L, Edeline V, Pacquement H, Michon J, Glorion C, et al. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004; 34:595–605. PMID: 15103428.
Article7. Wakabayashi H, Saito J, Taki J, Hashimoto N, Tsuchiya H, Gabata T, et al. Triple-phase contrast-enhanced MRI for the prediction of preoperative chemotherapeutic effect in patients with osteosarcoma: comparison with (99m)Tc-MIBI scintigraphy. Skeletal Radiol. 2016; 45:87–95. PMID: 26385785.
Article8. Lang P, Wendland MF, Saeed M, Gindele A, Rosenau W, Mathur A, et al. Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology. 1998; 206:227–235. PMID: 9423677.
Article9. Wang CS, Du LJ, Si MJ, Yin QH, Chen L, Shu M, et al. Noninvasive assessment of response to neoadjuvant chemotherapy in osteosarcoma of long bones with diffusion-weighted imaging: an initial in vivo study. PLoS One. 2013; 8:e72679. PMID: 23991141.
Article10. Byun BH, Kong CB, Lim I, Choi CW, Song WS, Cho WH, et al. Combination of 18F-FDG PET/CT and diffusion-weighted MR imaging as a predictor of histologic response to neoadjuvant chemotherapy: preliminary results in osteosarcoma. J Nucl Med. 2013; 54:1053–1059. PMID: 23670899.
Article11. Baunin C, Schmidt G, Baumstarck K, Bouvier C, Gentet JC, Aschero A, et al. Value of diffusion-weighted images in differentiating mid-course responders to chemotherapy for osteosarcoma compared to the histological response: preliminary results. Skeletal Radiol. 2012; 41:1141–1149. PMID: 22318350.
Article12. Bajpai J, Gamnagatti S, Kumar R, Sreenivas V, Sharma MC, Khan SA, et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol. 2011; 41:441–450. PMID: 20978754.
Article13. Oka K, Yakushiji T, Sato H, Hirai T, Yamashita Y, Mizuta H. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol. 2010; 39:141–146. PMID: 19924412.
Article14. Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol. 2006; 16:2637–2643. PMID: 16909220.
Article15. Yu J, Xu Q, Song JC, Li Y, Dai X, Huang DY, et al. The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2017; 27:1848–1857. PMID: 27631106.
Article16. Wang D, Li YH, Fu J, Wang H. Diffusion kurtosis imaging study on temporal lobe after nasopharyngeal carcinoma radiotherapy. Brain Res. 2016; 1648:387–393. PMID: 27514570.
Article17. Zhu L, Pan Z, Ma Q, Yang W, Shi H, Fu C, et al. Diffusion kurtosis imaging study of rectal adenocarcinoma associated with histopathologic prognostic factors: preliminary findings. Radiology. 2017; 284:66–76. PMID: 27929929.18. Bieling P, Rehan N, Winkler P, Helmke K, Maas R, Fuchs N, et al. Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol. 1996; 14:848–858. PMID: 8622033.
Article19. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982; 49:1221–1230. PMID: 6174200.20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article21. Uhl M, Saueressig U, Koehler G, Kontny U, Niemeyer C, Reichardt W, et al. Evaluation of tumour necrosis during chemotherapy with diffusion-weighted MR imaging: preliminary results in osteosarcomas. Pediatr Radiol. 2006; 36:1306–1311. PMID: 17031633.
Article22. Rosenkrantz AB, Padhani AR, Chenevert TL, Koh DM, De Keyzer F, Taouli B, et al. Body diffusion kurtosis imaging: basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging. 2015; 42:1190–1202. PMID: 26119267.
Article23. Le Bihan D. The ‘wet mind’: water and functional neuroimaging. Phys Med Biol. 2007; 52:R57–R90. PMID: 17374909.24. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005; 53:1432–1440. PMID: 15906300.
Article25. Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010; 23:698–710. PMID: 20632416.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion-Weighted Magnetic Resonance Imaging of Spine
- Emerging Techniques in Brain Tumor Imaging: What Radiologists Need to Know
- Histogram Analysis of Diffusion Kurtosis Magnetic Resonance Imaging for Diagnosis of Hepatic Fibrosis
- Squamous Cell Carcinoma of the Pancreas: A Case Report
- MR Spectroscopy and Diffusion Weighted Imaging Findings of Primary Non-Hodgkin Lymphoma of the Breast: Two Case Reports