J Rheum Dis.
2019 Apr;26(2):104-110. 10.4078/jrd.2019.26.2.104.
Causal Association between Bone Mineral Density and Osteoarthritis: A Mendelian Randomization Study
- Affiliations
-
- 1Department of Rheumatology, Korea University College of Medicine, Seoul, Korea. lyhcgh@korea.ac.kr
- KMID: 2442020
- DOI: http://doi.org/10.4078/jrd.2019.26.2.104
Abstract
OBJECTIVE
To examine whether bone mineral density (BMD) is causally associated with osteoarthritis (OA).
METHODS
We performed a two-sample Mendelian randomization (MR) analysis using the inverse-variance weighting (IVW), weighted median, and MR-Egger regression methods. We used publicly available summary statistics datasets of a genome-wide association study (GWAS) on femur neck (FN) BMD of individuals of European ancestry as the exposure and a GWAS for non-cancer illness code self-reported: OA from the individuals included in the UK Biobank as the outcome.
RESULTS
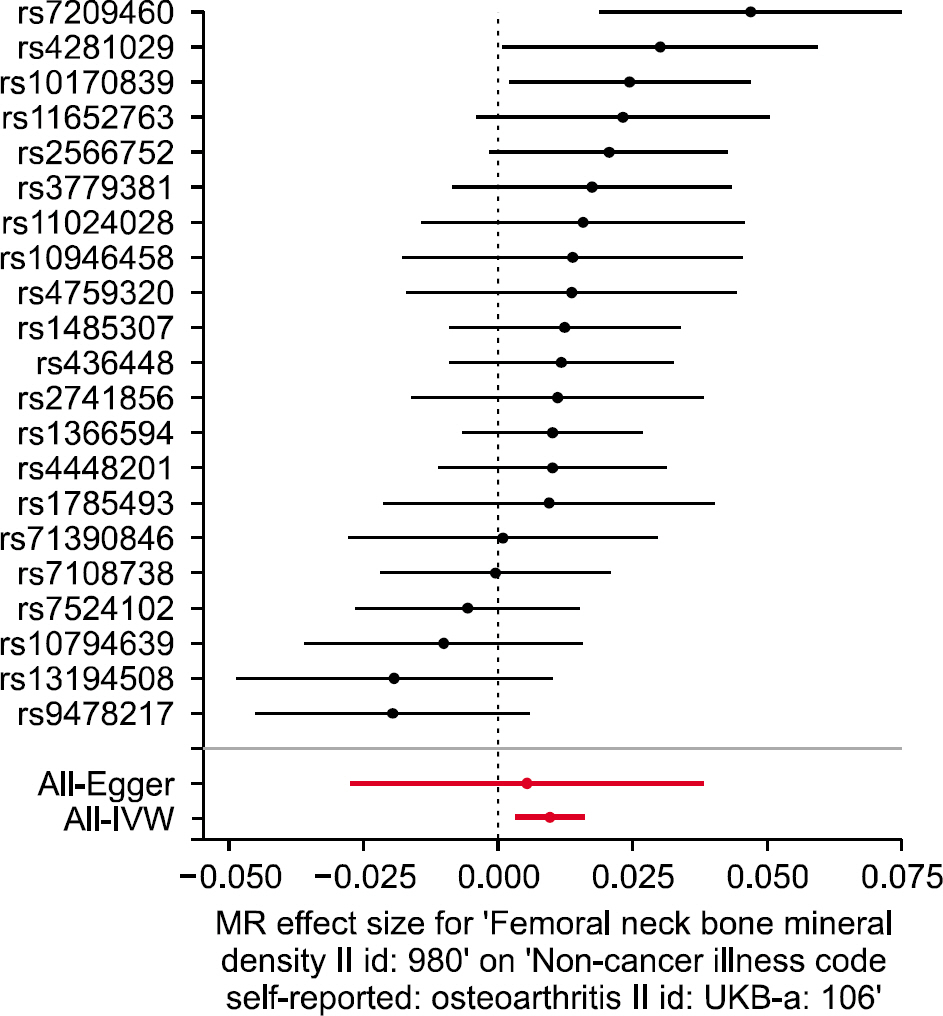
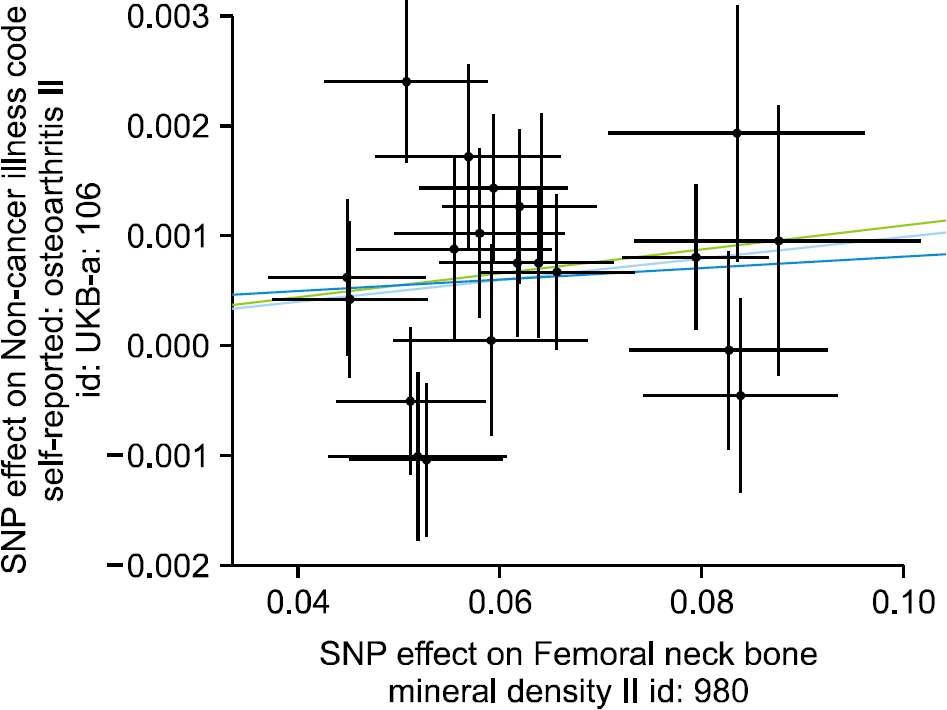
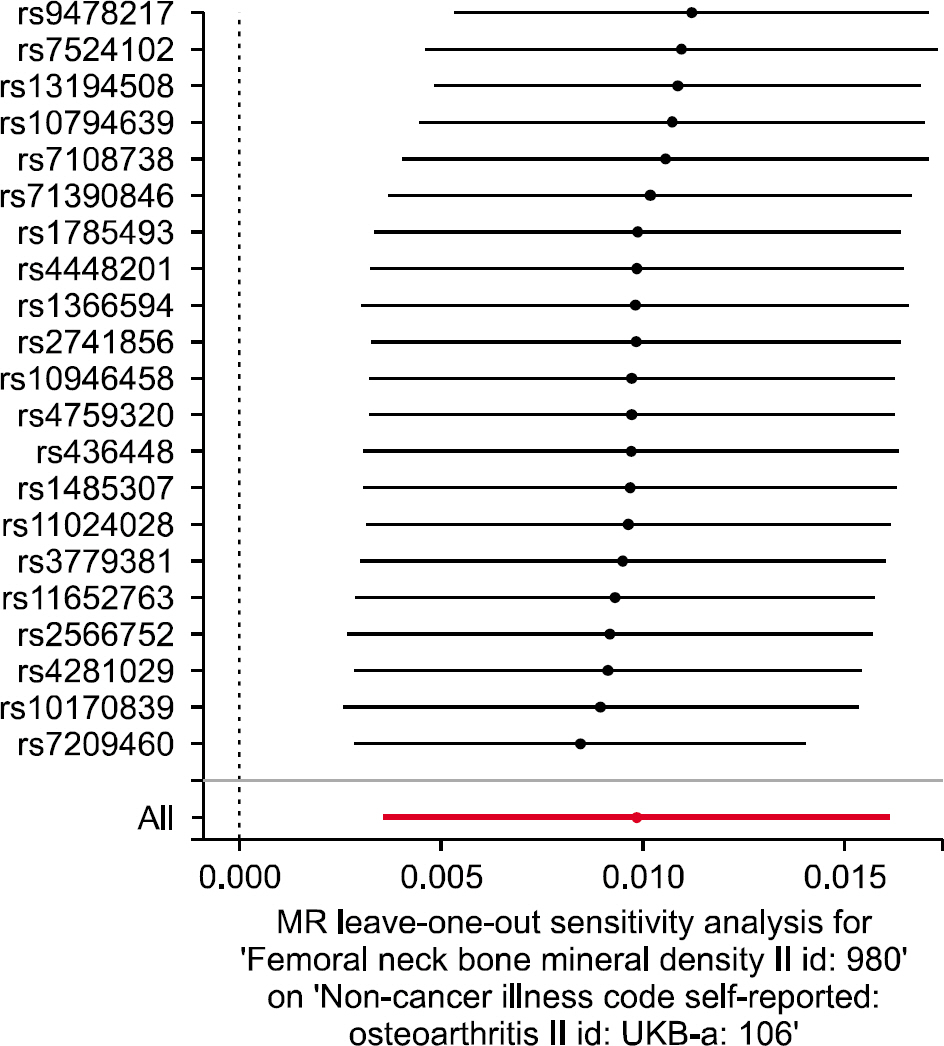
We selected 21 independent single-nucleotide polymorphisms with genome-wide significance (p<5.00E-08) from GWAS on FN BMD as the instrumental variables. The IVW method (beta=0.010, standard error [SE]=0.003, p=0.002) and the weighted median approach (beta=0.011, SE=0.004, p=0.006) yielded evidence of a causal association between FN BMD and OA. However, the MR-Egger analysis showed no causal association between FN BMD and OA (beta=0.005, SE=0.017, p=0.753). Since MR-Egger regression suffers from a lack of power and a susceptibility to weak instrument bias, the MR analysis results may support a causal association between FN BMD and OA.
CONCLUSION
The results of MR analysis by IVW and weighted median, but not MR-Egger regression indicate that FN BMD is likely to be causally associated with an increased risk of OA incidence The current findings may provide an opportunity to elucidate the underlying mechanisms of the effects of BMD on the OA incidence.
MeSH Terms
Figure
Cited by 1 articles
-
The Gut Microbiome and Osteoarthritis: A Two-Sample Mendelian Randomization Study
Young Ho Lee, Gwan Gyu Song
J Rheum Dis. 2021;28(2):94-100. doi: 10.4078/jrd.2021.28.2.94.
Reference
-
1. Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005; 365:965–73.
Article2. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013; 105:185–99.
Article3. Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015; 23:507–15.
Article4. Park EH, Kim JS, Lee JS, Lee YJ, Song YW, Lee EY. Compound K inhibits interleukin-1β-induced expression of inflammatory mediators and matrix metalloproteinases by inhibiting mitogen-activated protein kinase activation in chondrocytes. J Rheum Dis. 2018; 25:188–96.
Article5. Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford study. Arthritis Rheum. 2002; 46:92–9.
Article6. Nevitt MC, Zhang Y, Javaid MK, Neogi T, Curtis JR, Niu J, et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis. 2010; 69:163–8.
Article7. Ranstam J. Bias in observational studies. Acta Radiol. 2008; 49:644–5.
Article8. Burgess S, Daniel RM, Butterworth AS, Thompson SG. EPIC-InterAct Consortium. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2014; 44:484–95.
Article9. Lawlor DA. Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. 2016; 45:908–15.
Article10. Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello‐ Diez A, Leo PJ, et al. Whole‐ genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015; 526:112–7.11. Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genomewide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet. 2018; 50:549–58.
Article12. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet epidemiol. 2013; 37:658–65.
Article13. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. 2013; 178:1177–84.
Article14. Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the down-sides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016; 45:1717–26.
Article15. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015; 44:512–25.
Article16. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017; 32:377–89.
Article17. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016; 40:304–14.
Article18. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018; 7. pii: e34408.
Article19. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 315:1533–7.
Article20. Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016; 45:1961–74.21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.
Article22. Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK, et al. Radiographic osteoarthritis of the hip and bone mineral density. The study of osteoporotic fractures research group. Arthritis Rheum. 1995; 38:907–16.23. Smith GD, Ebrahim S. Mendelian randomization: genetic variants as instruments for strengthening causal inference in observational studies. Weinstein M, Vaupel JW, Wachter KW, editors. National Research Council (U.S.). Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys. National Research Council (U.S.). Committee on Population, 2006-2007. Biosocial surveys.Washington, D.C.: National Academies Press;2008.24. Thompson JR, Minelli C, Bowden J, Del Greco FM, Gill D, Jones EM, et al. Mendelian randomization incorporating uncertainty about pleiotropy. Stat Med. 2017; 36:4627–45.
Article25. Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004; 33:30–42.
Article26. Lo GH, Niu J, McLennan CE, Kiel DP, McLean RR, Guermazi A, et al. Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthritis Cartilage. 2008; 16:261–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Uric Acid and Gout have No Direct Causality With Osteoarthritis: A Mendelian Randomization Study
- Effects of bone metabolism on hematopoiesis: A Mendelian randomization study
- The Gut Microbiome and Osteoarthritis: A Two-Sample Mendelian Randomization Study
- Causal association between serum bilirubin and ischemic stroke: multivariable Mendelian randomization
- Exploration of errors in variance caused by using the first-order approximation in Mendelian randomization