Influence of Thyroid-stimulating Hormone Suppression Therapy on Bone Mineral Density in Patients with Differentiated Thyroid Cancer: A Meta-analysis
- Affiliations
-
- 1Department of Orthopaedic Surgery, Inje University College of Medicine, Seoul Paik Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea.
- 3Division of Gastroenterology, Department of Internal Medicine, Center for Cancer Prevention and Detection, National Cancer Center, Goyang, Korea.
- 4Department of Urology, Urological Cancer Center, National Cancer Center, Goyang, Korea.
- 5Department of Orthopaedic Surgery, Seoul National University Bundang Hospital, Seongnam, Korea. ykleemd@gmail.com
- KMID: 2440646
- DOI: http://doi.org/10.11005/jbm.2019.26.1.51
Abstract
- BACKGROUND
The effects of subclinical hyperthyroidism on bone mineral density (BMD) induced by thyroid-stimulating hormone (TSH) suppression therapy in patients with differentiated thyroid cancer (DTC) remains unclear. We conducted a meta-analysis to determine the influence of TSH suppression therapy on BMD.
METHODS
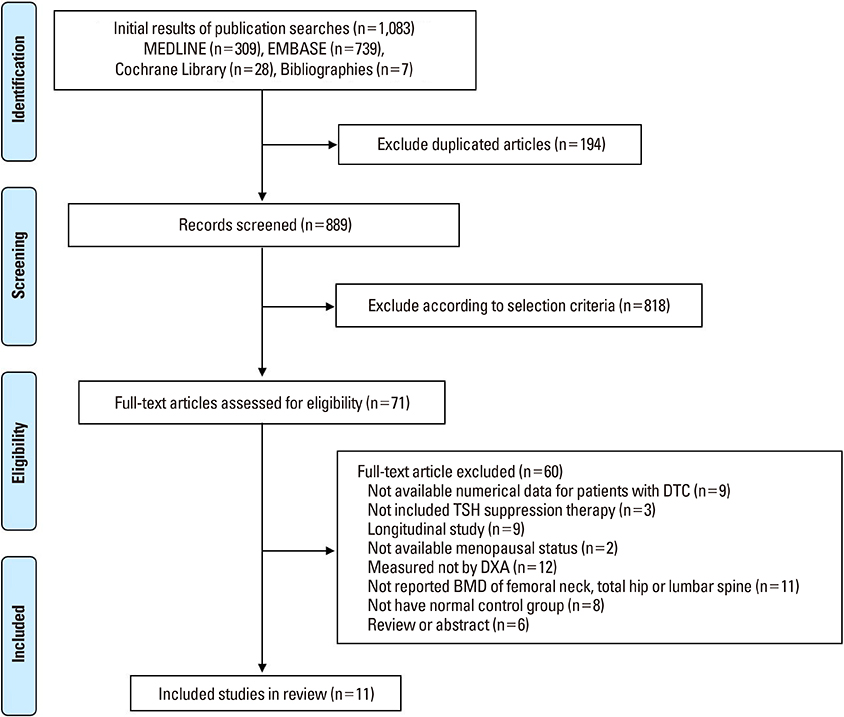
We performed a systematic search to identify studies which included BMD measurement of femoral neck, total hip or lumbar spine in patients on TSH suppression therapy for DTC. Main outcome measures were difference of BMD of femoral neck, total hip or lumbar spine measured by dual energy X-ray absorptiometry between patients and controls.
RESULTS
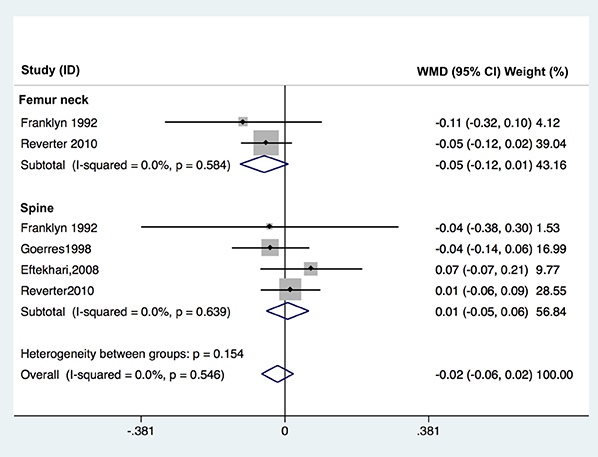
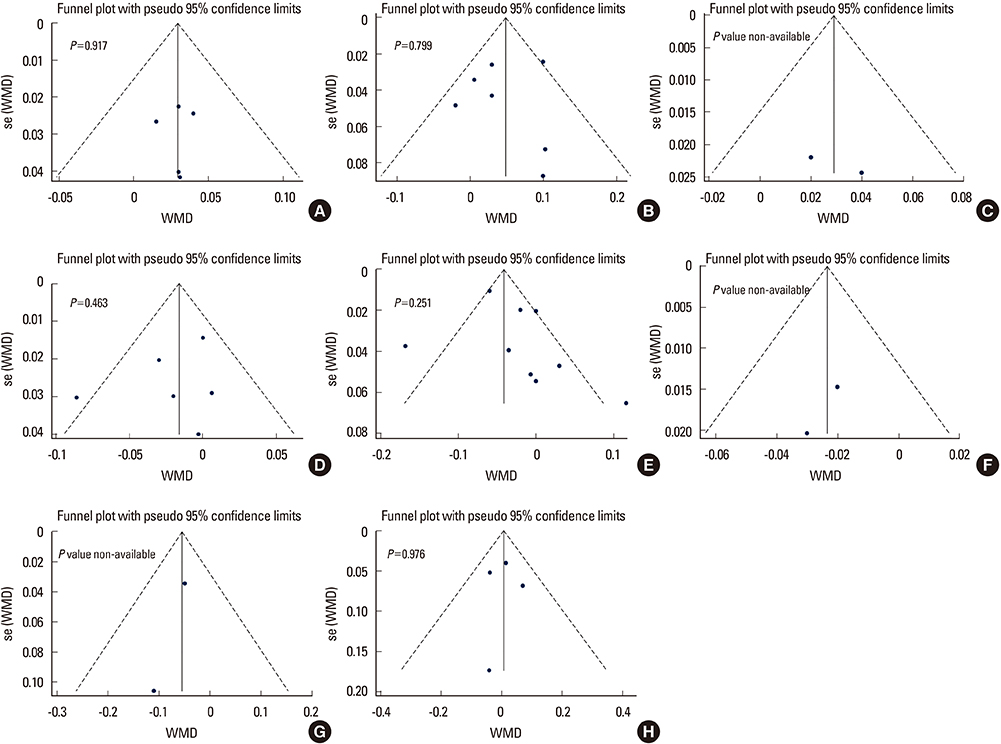
A systematic search yielded a total of 11 published controlled cross-sectional studies (including about 571 patients and 836 controls). TSH suppression therapy was associated with the lower BMD of total hip (weighted mean difference [WMD], −0.023; 95% confidence interval [CI], −0.047 to 0.000; P=0.050) and spine (WMD, −0.041; 95% CI, −0.057 to −0.026; P < 0.001) in postmenopausal women with DTC, while it was not associated with that in premenopausal women and men with DTC.
CONCLUSIONS
Although the included studies were limited by small numbers, results suggested possible association between chronic TSH suppression therapy and the lower BMD of spine and total hip in postmenopausal women (but not in premenopausal women and men) with DTC. A large, well-designed study with long-term follow-up would provide further insight into the influence of TSH suppression therapy and loss of BMD.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Evaluation and Management of Bone Health in Patients with Thyroid Diseases: a Position Statement from the Korean Thyroid Association
A Ram Hong, Hwa Young Ahn, Bu Kyung Kim, Seong Hee Ahn, So Young Park, Min-Hee Kim, Jeongmin Lee, Sun Wook Cho, Ho-Cheol Kang
Int J Thyroidol. 2022;15(1):1-16. doi: 10.11106/ijt.2022.15.1.1.Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association
A Ram Hong, Ho-Cheol Kang
Endocrinol Metab. 2023;38(2):175-189. doi: 10.3803/EnM.2023.1701.Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part II. Follow-up Surveillance after Initial Treatment 2024
Mijin Kim, Ji-In Bang, Ho-Cheol Kang, Sun Wook Kim, Dong Gyu Na, Young Joo Park, Youngduk Seo, Young Shin Song, So Won Oh, Sang-Woo Lee, Eun Kyung Lee, Ji Ye Lee, Dong-Jun Lim, Ari Chong, Yun Jae Chung, Chae Moon Hong, Min Kyoung Lee, Bo Hyun Kim
Int J Thyroidol. 2024;17(1):115-146. doi: 10.11106/ijt.2024.17.1.115.
Reference
-
1. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017; 317:1338–1348.
Article2. Mirian C, Grønhøj C, Jensen DH, et al. Trends in thyroid cancer: retrospective analysis of incidence and survival in Denmark 1980–2014. Cancer Epidemiol. 2018; 55:81–87.
Article3. Tam S, Boonsripitayanon M, Amit M, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid. 2018; 28:1301–1310.
Article4. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001; 86:1447–1463.
Article5. Williams GR, Bassett JHD. Thyroid diseases and bone health. J Endocrinol Invest. 2018; 41:99–109.
Article6. Haugen BR. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017; 123:372–381.
Article7. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–428.
Article8. Goretzki PE, Frilling A, Simon D, et al. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 1990; 118:48–63.
Article9. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998; 8:737–744.
Article10. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002; 34:554–564.
Article11. Franklyn JA, Betteridge J, Daykin J, et al. Long-term thyroxine treatment and bone mineral density. Lancet. 1992; 340:9–13.
Article12. Quan ML, Pasieka JL, Rorstad O. Bone mineral density in well-differentiated thyroid cancer patients treated with suppressive thyroxine: a systematic overview of the literature. J Surg Oncol. 2002; 79:62–69.
Article13. Biondi B, Palmieri EA, Fazio S, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab. 2000; 85:4701–4705.
Article14. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002; 87:968–974.
Article15. Napoli R, Biondi B, Guardasole V, et al. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation. 2001; 104:3076–3080.
Article16. Sgarbi JA, Villaça FG, Garbeline B, et al. The effects of early antithyroid therapy for endogenous subclinical hyperthyroidism in clinical and heart abnormalities. J Clin Endocrinol Metab. 2003; 88:1672–1677.
Article17. Smit JW, Eustatia-Rutten CF, Corssmit EP, et al. Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2005; 90:6041–6047.
Article18. Schlumberger M, Pacini F, Wiersinga WM, et al. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004; 151:539–548.
Article19. Rosario PW, Carvalho M, Calsolari MR. Symptoms of thyrotoxicosis, bone metabolism and occult atrial fibrillation in older women with mild endogenous subclinical hyperthyroidism. Clin Endocrinol (Oxf). 2016; 85:132–136.
Article20. Karunakaran P, Maharajan C, Chockalingam R, et al. The effect of total thyroidectomy on the recovery of bone mineral density in subjects with hyperthyroidism. Surgery. 2019; 165:80–84.
Article21. Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999; 130:750–758.
Article22. Tuchendler D, Bolanowski M. The influence of thyroid dysfunction on bone metabolism. Thyroid Res. 2014; 7:12.
Article23. Williams GR. Actions of thyroid hormones in bone. Endokrynol Pol. 2009; 60:380–388.24. Eriksen EF, Mosekilde L, Melsen F. Trabecular bone remodeling and bone balance in hyperthyroidism. Bone. 1985; 6:421–428.
Article25. Mosekilde L, Melsen F, Bagger JP, et al. Bone changes in hyperthyroidism: interrelationships between bone morphometry, thyroid function and calcium-phosphorus metabolism. Acta Endocrinol (Copenh). 1977; 85:515–525.
Article26. Abrahamsen B, Jørgensen HL, Laulund AS, et al. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. J Bone Miner Res. 2015; 30:898–905.
Article27. Gürlek A, Gedik O. Effect of endogenous subclinical hyperthyroidism on bone metabolism and bone mineral density in premenopausal women. Thyroid. 1999; 9:539–543.
Article28. Kim CW, Hong S, Oh SH, et al. Change of bone mineral density and biochemical markers of bone turnover in patients on suppressive levothyroxine therapy for differentiated thyroid carcinoma. J Bone Metab. 2015; 22:135–141.
Article29. Stěpán JJ, Límanová Z. Biochemical assessment of bone loss in patients on long-term thyroid hormone treatment. Bone Miner. 1992; 17:377–388.
Article30. Jódar E, Martínez-Díaz-Guerra G, Azriel S, et al. Bone mineral density in male patients with L-thyroxine suppressive therapy and Graves disease. Calcif Tissue Int. 2001; 69:84–87.
Article31. Heijckmann AC, Huijberts MS, Geusens P, et al. Hip bone mineral density, bone turnover and risk of fracture in patients on long-term suppressive L-thyroxine therapy for differentiated thyroid carcinoma. Eur J Endocrinol. 2005; 153:23–29.
Article32. Jódar E, Begoña López M, García L, et al. Bone changes in pre- and postmenopausal women with thyroid cancer on levothyroxine therapy: evolution of axial and appendicular bone mass. Osteoporos Int. 1998; 8:311–316.
Article33. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 350:g7647.
Article34. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605.
Article35. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.
Article36. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.
Article37. Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988; 41:257–278.
Article38. Tournis S, Antoniou JD, Liakou CG, et al. Volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in women with differentiated thyroid cancer under TSH suppression. Clin Endocrinol (Oxf). 2015; 82:197–204.
Article39. Toivonen J, Tähtelä R, Laitinen K, et al. Markers of bone turnover in patients with differentiated thyroid cancer with and following withdrawal of thyroxine suppressive therapy. Eur J Endocrinol. 1998; 138:667–673.
Article40. Reverter JL, Holgado S, Alonso N, et al. Lack of deleterious effect on bone mineral density of long-term thyroxine suppressive therapy for differentiated thyroid carcinoma. Endocr Relat Cancer. 2005; 12:973–981.
Article41. Reverter JL, Colomé E, Holgado S, et al. Bone mineral density and bone fracture in male patients receiving long-term suppressive levothyroxine treatment for differentiated thyroid carcinoma. Endocrine. 2010; 37:467–472.
Article42. Moon JH, Jung KY, Kim KM, et al. The effect of thyroid stimulating hormone suppressive therapy on bone geometry in the hip area of patients with differentiated thyroid carcinoma. Bone. 2016; 83:104–110.
Article43. Kung AW, Lorentz T, Tam SC. Thyroxine suppressive therapy decreases bone mineral density in post-menopausal women. Clin Endocrinol (Oxf). 1993; 39:535–540.
Article44. Hawkins F, Rigopoulou D, Papapietro K, et al. Spinal bone mass after long-term treatment with L-thyroxine in postmenopausal women with thyroid cancer and chronic lymphocytic thyroiditis. Calcif Tissue Int. 1994; 54:16–19.
Article45. Goerres G, Theiler R, Müller-Brand J. Interfemur variation of bone mineral density in patients receiving high-dose thyroxin therapy. Calcif Tissue Int. 1998; 63:98–101.
Article46. Giannini S, Nobile M, Sartori L, et al. Bone density and mineral metabolism in thyroidectomized patients treated with long-term L-thyroxine. Clin Sci (Lond). 1994; 87:593–597.
Article47. Eftekhari M, Asadollahi A, Beiki D, et al. The long term effect of levothyroxine on bone mineral density in patients with well differentiated thyroid carcinoma after treatment. Hell J Nucl Med. 2008; 11:160–163.48. Lee JS, Buzková P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med. 2010; 170:1876–1883.
Article49. Stall GM, Harris S, Sokoll LJ, et al. Accelerated bone loss in hypothyroid patients overtreated with L-thyroxine. Ann Intern Med. 1990; 113:265–269.
Article50. Adlin EV, Maurer AH, Marks AD, et al. Bone mineral density in postmenopausal women treated with L-thyroxine. Am J Med. 1991; 90:360–366.
Article51. Greenspan SL, Greenspan FS, Resnick NM, et al. Skeletal integrity in premenopausal and postmenopausal women receiving long-term L-thyroxine therapy. Am J Med. 1991; 91:5–14.
Article52. Paul TL, Kerrigan J, Kelly AM, et al. Long-term L-thyroxine therapy is associated with decreased hip bone density in premenopausal women. JAMA. 1988; 259:3137–3141.
Article53. Recker DP, Shapiro B. The effect of thyroidectomy on bone mineral content in perimenopausal women. Thyroidology. 1989; 1:59–65.54. Ross DS, Neer RM, Ridgway EC, et al. Subclinical hyperthyroidism and reduced bone density as a possible result of prolonged suppression of the pituitary-thyroid axis with L-thyroxine. Am J Med. 1987; 82:1167–1170.
Article55. Melton LJ 3rd, Ardila E, Crowson CS, et al. Fractures following thyroidectomy in women: a population-based cohort study. Bone. 2000; 27:695–700.
Article56. Lin SY, Lin CL, Chen HT, et al. Risk of osteoporosis in thyroid cancer patients using levothyroxine: a population-based study. Curr Med Res Opin. 2018; 34:805–812.
Article57. Diab DL, Watts NB. Postmenopausal osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2013; 20:501–509.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: a Position Statement from the Korean Thyroid Association
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association
- Long-Term Effect of Prolonged TSH Suppression on the Skeletal System
- TSH Suppression after Differentiated Thyroid Cancer Surgery and Osteoporosis
- Bone Mineral Density in Thyroid Cancer Patients: Data from the Korea National Health and Nutrition Examination Survey