Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: a real world intention-to-treat analysis
- Affiliations
-
- 1Department of Gastroenterology, Dayanand Medical College & Hospital, Ludhiana, India. ajitsood10@gmail.com
- 2School of Biotechnology, Jawaharlal Nehru University, New Delhi, India.
- 3Department of Internal Medicine, Dayanand Medical College & Hospital, Ludhiana, India.
- 4Multidisciplinary Centre for Advanced Research and Studies (MCARS), Jamia Millia Islamia, New Delhi, India.
- 5Department of Pathology, Dayanand Medical College & Hospital, Ludhiana, India.
- 6Department of Pharmacology, Dayanand Medical College & Hospital, Ludhiana, India.
- KMID: 2438446
- DOI: http://doi.org/10.5217/ir.2018.00089
Abstract
- BACKGROUND/AIMS
Four high-quality randomized controlled trials have proven the efficacy of fecal microbiota transplantation (FMT) in active ulcerative colitis (UC). We assessed the efficacy of FMT in a real-world setting involving steroid-dependent patients with UC.
METHODS
This was a single-center prospective analysis of data from steroid-dependent patients with UC treated with FMT from September 2015 to September 2017 at the Dayanand Medical College, a tertiary care center in India. Fecal samples from random unrelated donors were administered through colonoscopy at weeks 0, 2, 6, 10, 14, 18, and 22. The primary outcome was achievement of steroid-free clinical remission, and the secondary outcomes were clinical response and endoscopic remission at 24 weeks. Modified intention-to-treat analysis was performed, which included subjects who underwent at least 1 FMT.
RESULTS
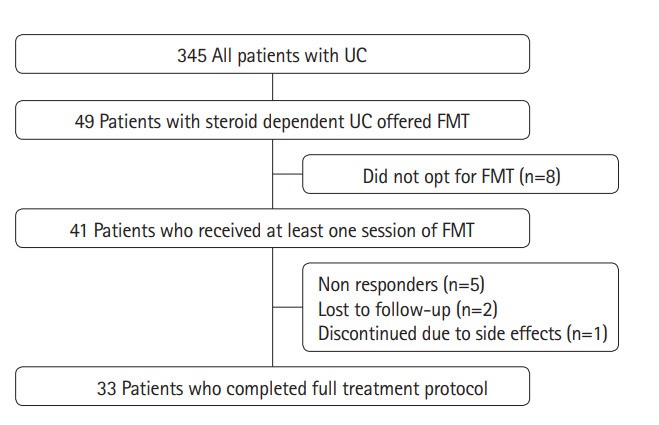
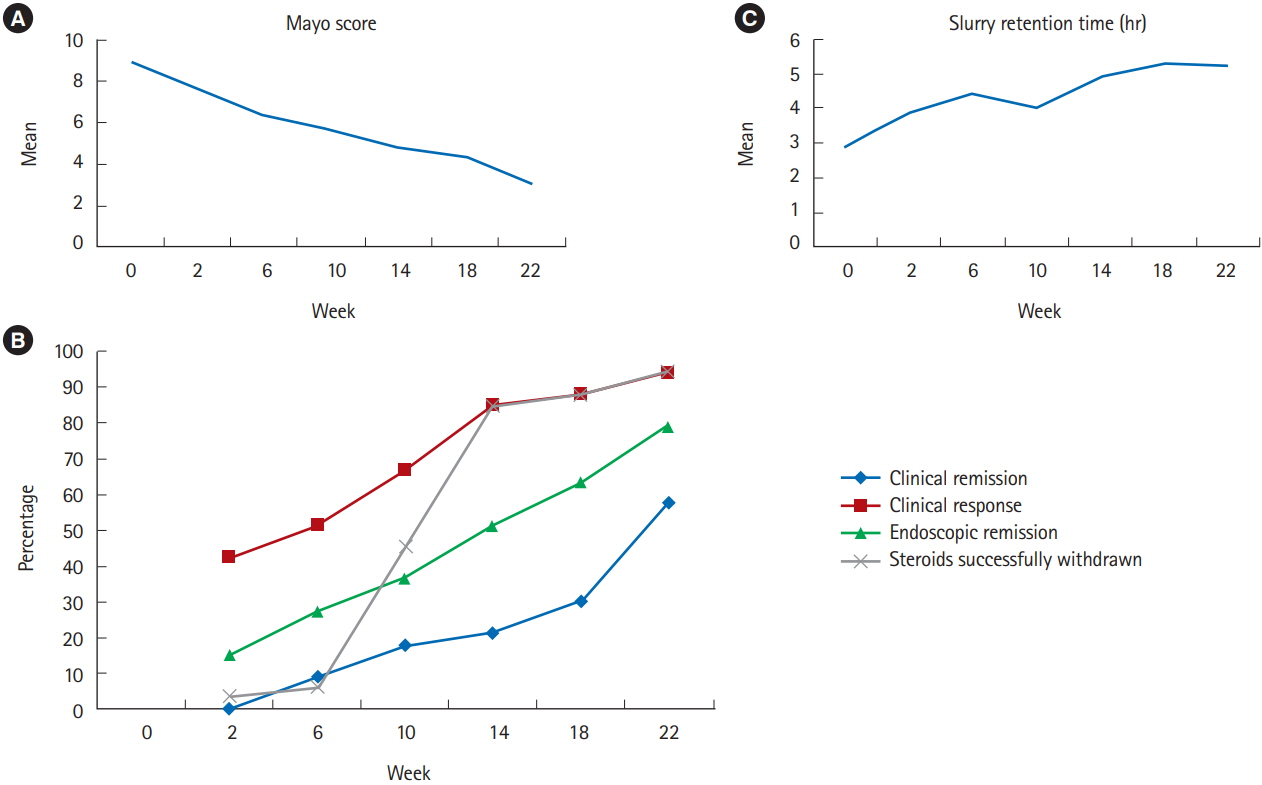
Of 345 patients with UC treated during the study period, 49 (14.2%) had steroid-dependent UC. Of these 49 patients, 41 underwent FMT: 33 completed 7 sessions over 22 weeks according to the protocol, and 8 discontinued treatment (non-response, 5; lost to follow-up, 2; and fear of adverse effects, 1). At week 24, steroid-free clinical remission was achieved in 19 out of 41 (46.3%) patients, whereas clinical response and endoscopic remission were achieved in 31 out of 41 (75.6%) and 26 out of 41 (63.4%) patients, respectively. All patients with clinical response were able to withdraw steroids. There were no serious adverse events necessitating discontinuation.
CONCLUSIONS
A multisession FMT via the colonoscopic route is a promising therapeutic option for patients with steroid-dependent UC, as it can induce clinical remission and aid in steroid withdrawal.
MeSH Terms
Figure
Cited by 6 articles
-
Efficacy and Safety of Fecal Microbiota Transplantation and Prospect of Microbe-based Therapies for Inflammatory Bowel Disease
Hoon Gil Jo, Geom Seog Seo
Korean J Gastroenterol. 2021;78(1):31-36. doi: 10.4166/kjg.2021.089.Incidental benefits after fecal microbiota transplant for ulcerative colitis
Ramit Mahajan, Vandana Midha, Arshdeep Singh, Varun Mehta, Yogesh Gupta, Kirandeep Kaur, Ritu Sudhakar, Anmol Singh Pannu, Dharmatma Singh, Ajit Sood
Intest Res. 2020;18(3):337-340. doi: 10.5217/ir.2019.00108.Fecal microbiota transplantation for induction of remission, maintenance and rescue in patients with corticosteroid-dependent ulcerative colitis: a long-term follow-up real-world cohort study
Avnish Kumar Seth, Priti Jain
Intest Res. 2022;20(2):251-259. doi: 10.5217/ir.2021.00069.Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
Young-Seok Cho
Intest Res. 2019;17(1):6-8. doi: 10.5217/ir.2018.00171.Physician education can minimize inappropriate steroid use in patients with inflammatory bowel disease: the ACTION study
Yehyun Park, Chang Hwan Choi, Hyun Soo Kim, Hee Seok Moon, Do Hyun Kim, Jin Ju Kim, Dennis Teng, Dong Il Park
Intest Res. 2022;20(4):452-463. doi: 10.5217/ir.2021.00125.The practice of fecal microbiota transplantation in inflammatory bowel disease
Umang Arora, Saurabh Kedia, Vineet Ahuja
Intest Res. 2024;22(1):44-64. doi: 10.5217/ir.2023.00085.
Reference
-
1. Ekbom A, Helmick CG, Zack M, Holmberg L, Adami HO. Survival and causes of death in patients with inflammatory bowel disease: a population-based study. Gastroenterology. 1992; 103:954–960.
Article2. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002; 50:485–489.
Article3. Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006; 55:47–53.
Article4. Adler DJ, Korelitz BI. The therapeutic efficacy of 6-mercaptopurine in refractory ulcerative colitis. Am J Gastroenterol. 1990; 85:717–722.5. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article6. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146:392–400.
Article7. Armuzzi A, Pugliese D, Danese S, et al. Infliximab in steroiddependent ulcerative colitis: effectiveness and predictors of clinical and endoscopic remission. Inflamm Bowel Dis. 2013; 19:1065–1072.8. Girotra M, Garg S, Anand R, Song Y, Dutta SK. Fecal microbiota transplantation for recurrent Clostridium difficile infection in the elderly: long-term outcomes and microbiota changes. Dig Dis Sci. 2016; 61:3007–3015.
Article9. Ren R, Sun G, Yang Y, et al. A pilot study of treating ulcerative colitis with fecal microbiota transplantation. Zhonghua Nei Ke Za Zhi. 2015; 54:411–415.10. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014; 8:1569–1581.
Article11. Uygun A, Ozturk K, Demirci H, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine (Baltimore). 2017; 96:e6479.
Article12. Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013; 56:597–601.
Article13. Suskind DL, Singh N, Nielson H, Wahbeh G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2015; 60:27–29.
Article14. Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013; 108:1620–1630.
Article15. Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015; 13:298.
Article16. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.
Article17. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–118.
Article18. Costello SP, Waters O, Bryant RV, et al. Short duration, low intensity, pooled fecal microbiota transplantation induces remission in patients with mild-moderately active ulcerative colitis: a randomised controlled trial. Gastroenterology. 2017; 152:S198–S199.
Article19. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017; 389:1218–1228.
Article20. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article21. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008; 14:1660–1666.
Article22. Narula N, Kassam Z, Yuan Y, et al. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. 2017; 23:1702–1709.23. Fairhurst NG, Travis SP. Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis? Intest Res. 2018; 16:209–215.
Article24. Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014; 146:1547–1553.
Article25. Serban DE. Microbiota in inflammatory bowel disease pathogenesis and therapy: is it all about diet? Nutr Clin Pract. 2015; 30:760–779.
Article26. Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012; 107:1452–1459.
Article27. Walker AW, Sanderson JD, Churcher C, et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and noninflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011; 11:7.
Article28. Agrawal M, Aroniadis OC, Brandt LJ, et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol. 2016; 50:403–407.
Article29. Vermeire S, Joossens M, Verbeke K, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis. 2016; 10:387–394.
Article30. Xu L, Zhang T, Cui B, et al. Clinical efficacy maintains patients’ positive attitudes toward fecal microbiota transplantation. Medicine (Baltimore). 2016; 95:e4055.
Article31. Damman CJ, Brittnacher MJ, Westerhoff M, et al. Low level engraftment and improvement following a single colonoscopic administration of fecal microbiota to patients with ulcerative colitis. PLoS One. 2015; 10:e0133925.
Article32. Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008; 3:417–427.
Article33. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006; 55:205–211.
Article34. Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006; 44:4136–4141.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
- Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis?
- Coordinated Hospital-Home Fecal Microbiota Transplantation via Percutaneous Endoscopic Cecostomy for Recurrent Steroid-Dependent Ulcerative Colitis
- Current new challenges in the management of ulcerative colitis
- Fecal microbiota transplantation for induction of remission, maintenance and rescue in patients with corticosteroid-dependent ulcerative colitis: a long-term follow-up real-world cohort study