Intest Res.
2022 Apr;20(2):251-259. 10.5217/ir.2021.00069.
Fecal microbiota transplantation for induction of remission, maintenance and rescue in patients with corticosteroid-dependent ulcerative colitis: a long-term follow-up real-world cohort study
- Affiliations
-
- 1Department of Gastroenterology and Hepatobiliary Sciences, Fortis Memorial Research Institute, Gurugram, India
- 2Department of Histopathology, Fortis Memorial Research Institute, Gurugram, India
- KMID: 2529571
- DOI: http://doi.org/10.5217/ir.2021.00069
Abstract
- Background/Aims
To study role of fecal microbiota transplantation (FMT) in induction, maintenance, and rescue in patients with corticosteroid-dependent ulcerative colitis (CDUC).
Methods
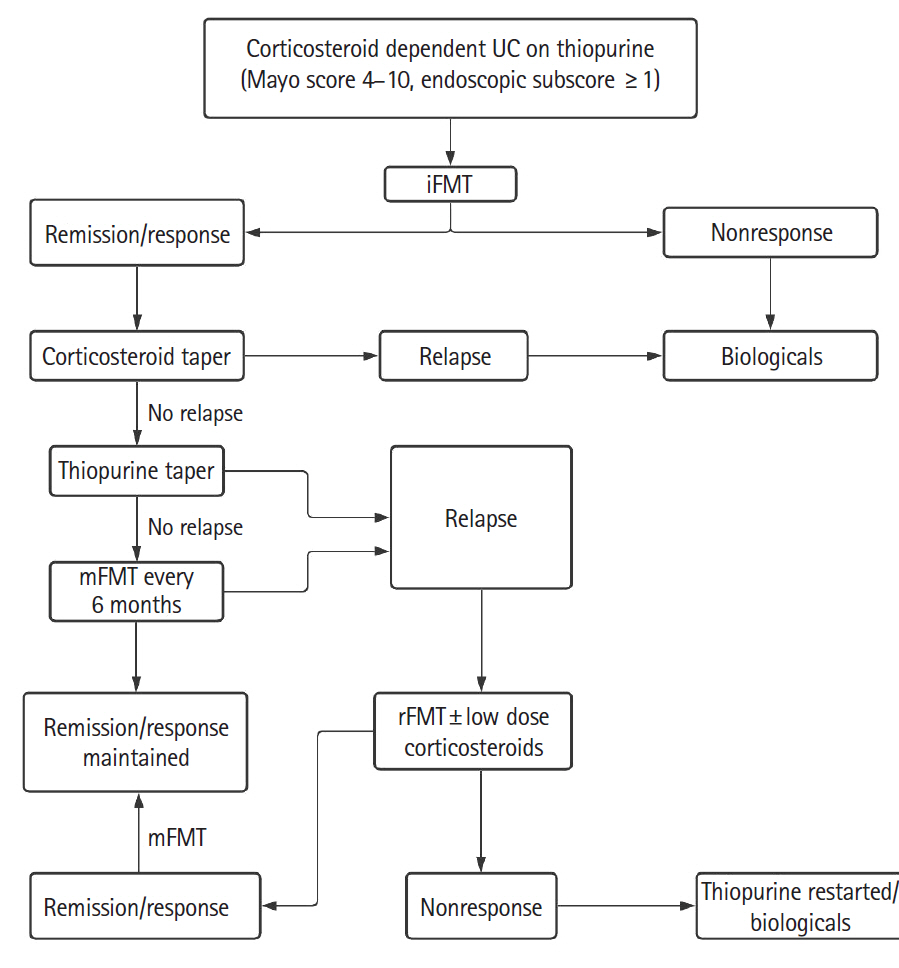
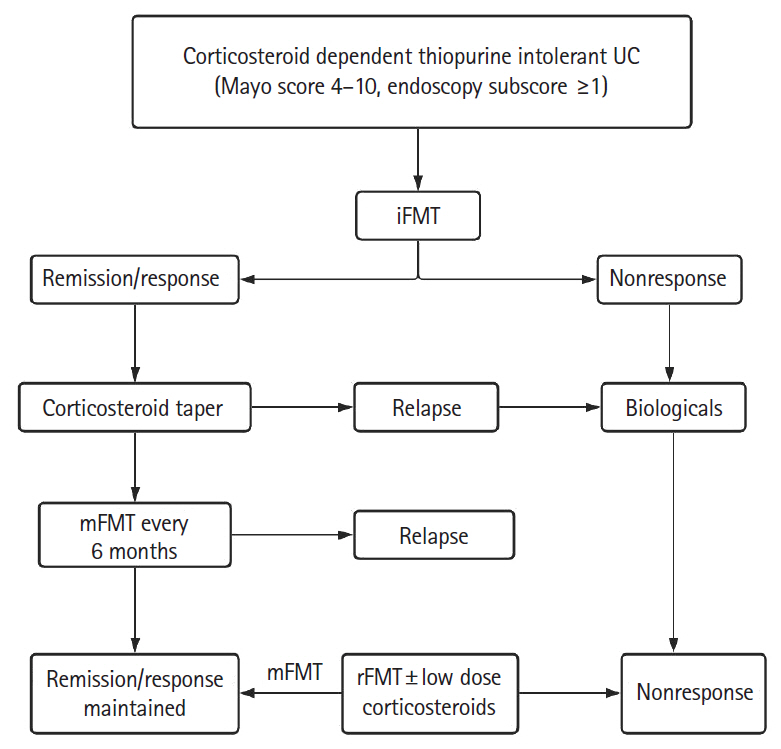
Patients with active CDUC received 3 fortnightly sessions of colonoscopic induction FMT (iFMT) in addition to standard of care. In patients who achieved clinical remission (CR) or response, prednisolone was tapered from week 4 and azathioprine from week 12. Responders were advised maintenance FMT (mFMT) every 6 months. Those with relapse were offered rescue FMT (rFMT), and low dose prednisolone was added if there was no improvement in 2 weeks.
Results
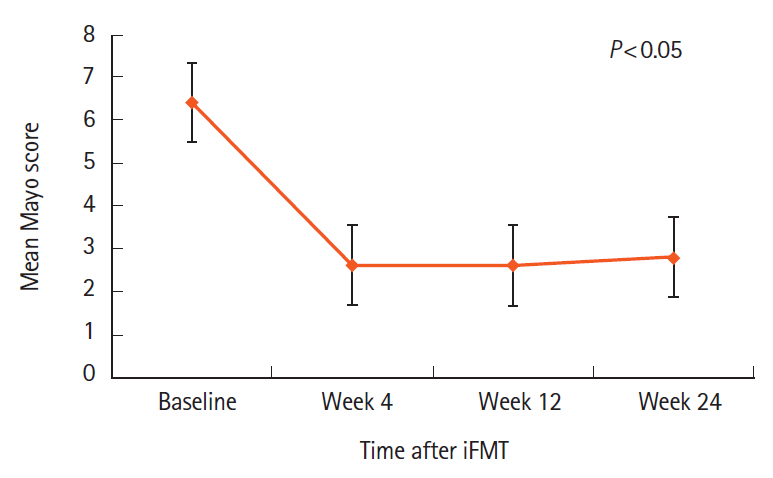
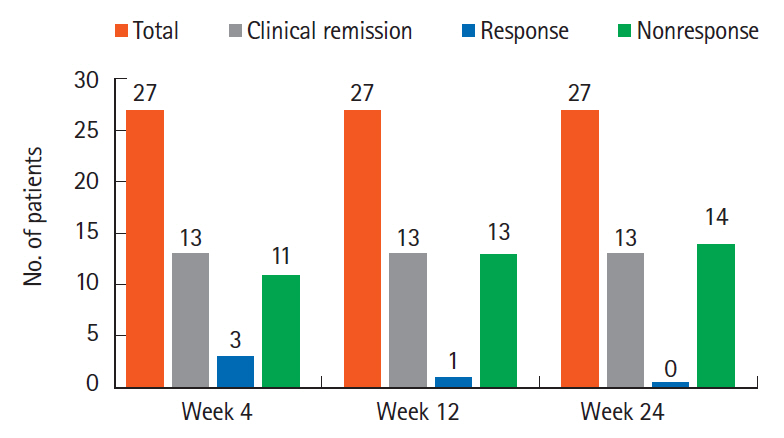
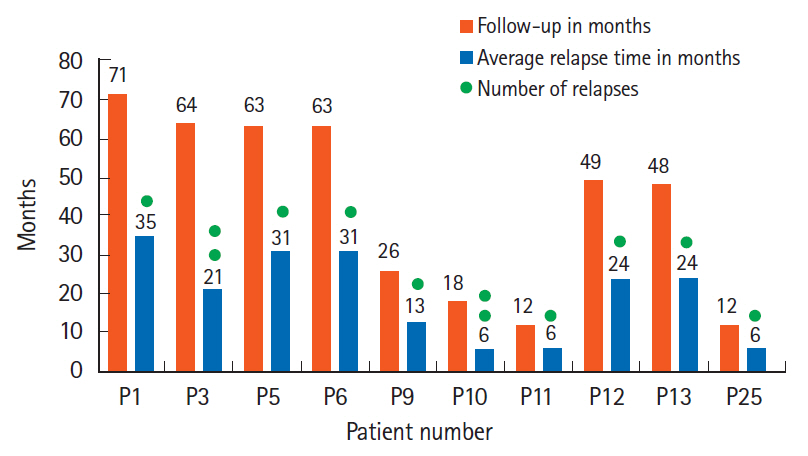
All 27 patients enrolled completed iFMT and were followed up for 39 months (range, 9–71 months). The mean Mayo score decreased from 6.4±2.5 at baseline to 2.6±3.7 at week 4, 2.6±3.4 at week 12, and 2.8±3.8 at week 24 (P<0.05). Corticosteroid-free CR and clinical response at week 12 were seen in 13 patients (48%) and 1 patient (3.7%), respectively. Corticosteroid and azathioprine-free CR at week 24 was seen in 13 patients (48%) and in them histological response was seen in 2 patients (15.2%) at week 4, 5 patients (38.4%) at week 12, and 10 patients (76.9%) at week 24. First relapse was seen in 10 of 13 responders (76.9%) at a median of 14.8 months (range, 6–34 months) after iFMT and was less frequent in patients on mFMT. Relapse was treated successfully with rFMT alone in 4 patients (40%) and rFMT with low dose steroids in 5 patients (50%).
Conclusions
iFMT, mFMT, and rFMT may have a role in treatment of selected patients with CDUC.
Figure
Cited by 1 articles
-
The practice of fecal microbiota transplantation in inflammatory bowel disease
Umang Arora, Saurabh Kedia, Vineet Ahuja
Intest Res. 2024;22(1):44-64. doi: 10.5217/ir.2023.00085.
Reference
-
1. Bejaoui M, Sokol H, Marteau P. Targeting the microbiome in inflammatory bowel disease: critical evaluation of current concepts and moving to new horizons. Dig Dis. 2015; 33 Suppl 1:105–112.
Article2. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018; 66:e1–e48.3. Mcilroy JR, Nalagatla N, Hansen R, Hart A, Hold GL. Faecal microbiota transplantation as a treatment for inflammatory bowel disease: a national survey of adult and paediatric gastroenterologists in the UK. Frontline Gastroenterol. 2018; 9:250–255.
Article4. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article5. Seth AK, Rawal P, Bagga R, Jain P. Successful colonoscopic fecal microbiota transplantation for active ulcerative colitis: first report from India. Indian J Gastroenterol. 2016; 35:393–395.
Article6. Seth AK, Jain P. Colonoscopic fecal microbiota transplantation for patients with ulcerative colitis with failure of 5-amino salicylates and dependence on corticosteroids and/or thiopurine intolerance: 30-month follow up of first pilot study in India. Indian J Gastroenterol. 2017; 36:S1–S105.7. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol. 1989; 24:2–6.
Article8. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017; 66:569–580.
Article9. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000; 47:404–409.
Article10. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.
Article11. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–118.
Article12. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017; 389:1218–1228.
Article13. Crothers J, Kassam Z, Smith M, et al. A double-blind, randomized, placebo-control pilot trial of fecal microbiota transplantation capsules from rationally selected donors in active ulcerative colitis. Gastroenterology. 2018; 154:S-1050–S-1051.14. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019; 321:156–164.
Article15. Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017; 46:213–224.
Article16. Narula N, Kassam Z, Yuan Y, et al. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. 2017; 23:1702–1709.17. Lam WC, Zhao C, Ma WJ, Yao L. The clinical and steroid-free remission of fecal microbiota transplantation to patients with ulcerative colitis: a meta-analysis. Gastroenterol Res Pract. 2019; 2019:1287493.
Article18. Tang LL, Feng WZ, Cheng JJ, Gong YN. Clinical remission of ulcerative colitis after different modes of faecal microbiota transplantation: a meta-analysis. Int J Colorectal Dis. 2020; 35:1025–1034.
Article19. Sood A, Mahajan R, Juyal G, et al. Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: a real world intention-to-treat analysis. Intest Res. 2019; 17:78–86.
Article20. Garza-González E, Mendoza-Olazarán S, Morfin-Otero R, et al. Intestinal microbiome changes in fecal microbiota transplant (FMT) vs. FMT enriched with Lactobacillus in the treatment of recurrent Clostridioides difficile infection. Can J Gastroenterol Hepatol. 2019; 2019:4549298.21. Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis. 2019; 13:1311–1317.
Article22. Qazi T, Amaratunga T, Barnes EL, Fischer M, Kassam Z, Allegretti JR. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: systematic review and metaanalysis. Gut Microbes. 2017; 8:574–588.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
- Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: a real world intention-to-treat analysis
- Fecal Microbiota Transplantation to Patients with Refractory Very Early Onset Ulcerative Colitis
- Coordinated Hospital-Home Fecal Microbiota Transplantation via Percutaneous Endoscopic Cecostomy for Recurrent Steroid-Dependent Ulcerative Colitis
- Failure of Fecal Microbiota Transplantation in a Three-Year-Old Child with Severe Refractory Ulcerative Colitis