Pediatr Gastroenterol Hepatol Nutr.
2016 Sep;19(3):214-220. 10.5223/pghn.2016.19.3.214.
Failure of Fecal Microbiota Transplantation in a Three-Year-Old Child with Severe Refractory Ulcerative Colitis
- Affiliations
-
- 1Department of Pediatrics, Jichi Medical University, Shimotsuke, Japan. h-kumagai@jichi.ac.jp
- 2Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan.
- KMID: 2364782
- DOI: http://doi.org/10.5223/pghn.2016.19.3.214
Abstract
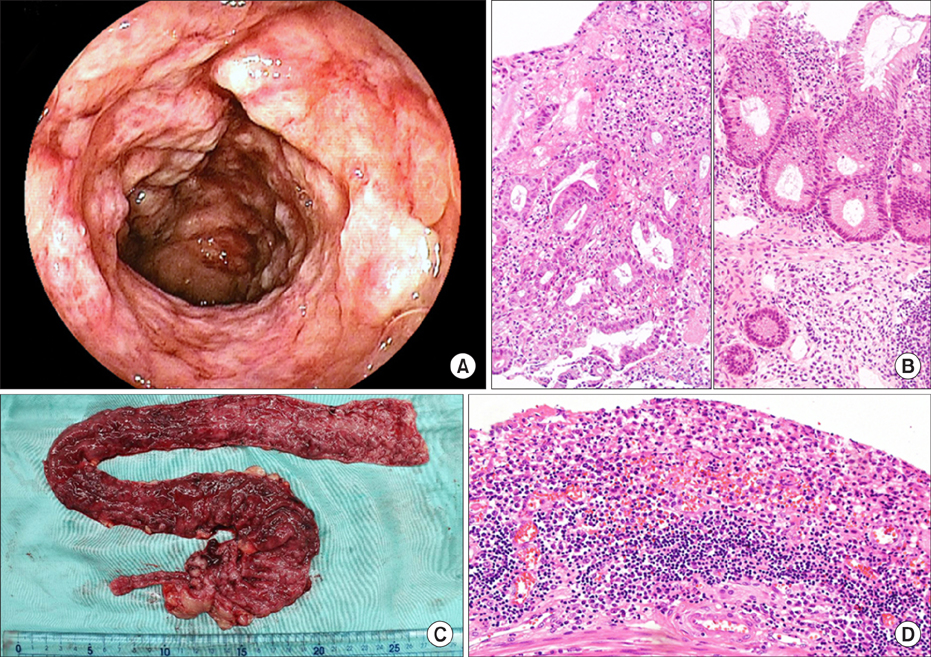
- Fecal microbiota transplantation (FMT) is a treatment designed to correct gut dysbiosis by administration of feces from a healthy volunteer. It is still unclear whether FMT for children with ulcerative colitis (UC) is effective or hazardous. Here we describe a young patient to have received FMT for UC. A three-year-old girl was admitted to our hospital with severe active UC, and treated with aminosalicylates and various immunosuppressive drugs. As remission was not achieved, we decided to try FMT before colectomy. We administered donor fecal material a total of six times by retention enema (×2) and via a nasoduodenal tube (×4) within 10 days. The patient developed abdominal pain and pyrexia after each FMT session. Analyses revealed the transferred donor fecal microbiota had not been retained by the patient, who ultimately underwent colectomy. The severity of the UC and/or timing of FMT may have partly accounted for the poor outcome.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Fecal Microbiota Transplantation to Patients with Refractory Very Early Onset Ulcerative Colitis
Toshifumi Yodoshi, Thomas L. Hurt
Pediatr Gastroenterol Hepatol Nutr. 2018;21(4):355-360. doi: 10.5223/pghn.2018.21.4.355.The practice of fecal microbiota transplantation in inflammatory bowel disease
Umang Arora, Saurabh Kedia, Vineet Ahuja
Intest Res. 2024;22(1):44-64. doi: 10.5217/ir.2023.00085.
Reference
-
1. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010; 464:59–65.
Article2. Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008; 298:463–472.
Article3. Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, Hanauer SB, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011; 106:Suppl 1. S2–S25.
Article4. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.
Article5. Kanai T, Mikami Y, Hayashi A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J Gastroenterol. 2015; 50:928–939.
Article6. Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, et al. Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011; 9:1044–1049.
Article7. Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K, et al. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol. 2002; 68:5445–5451.
Article8. Hayashi H, Sakamoto M, Benno Y. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol Immunol. 2002; 46:819–831.
Article9. Sakamoto M, Hayashi H, Benno Y. Terminal restriction fragment length polymorphism analysis for human fecal microbiota and its application for analysis of complex bifidobacterial communities. Microbiol Immunol. 2003; 47:133–142.
Article10. Kumagai H, Maisawa S, Tanaka M, Takahashi M, Takasago Y, Nishijima A, et al. Intestinal microbiota and secretory immunoglobulin A in feces of exclusively breast-fed infants with blood-streaked stools. Microbiol Immunol. 2012; 56:657–663.
Article11. Damman CJ, Brittnacher MJ, Westerhoff M, Hayden HS, Radey M, Hager KR, et al. Low level engraftment and improvement following a single colonoscopic administration of fecal microbiota to patients with ulcerative colitis. PLoS One. 2015; 10:e0133925.
Article12. Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013; 56:597–601.
Article13. Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015; 6:8292.
Article14. Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013; 108:1620–1630.
Article15. Vandenplas Y, Veereman G, van der Werff Ten Bosch J, Goossens A, Pierard D, Samsom JN, et al. Fecal microbial transplantation in early-onset colitis: caution advised. J Pediatr Gastroenterol Nutr. 2015; 61:e12–e14.16. Vandenplas Y, Pierard D, De Greef E. Fecal microbiota transplantation: just a fancy trend? J Pediatr Gastroenterol Nutr. 2015; 61:4–7.17. Vermeire S, Joossens M, Verbeke K, Hildebrand F, Machiels K, van den Broecket K, et al. Pilot study on the safety and efficacy of faecal microbiota transplantation in refractory Crohn's disease. Gastroenterol. 2012; 142:Suppl 1. S360.18. Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn's disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014; 8:252–253.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Microbiota Transplantation to Patients with Refractory Very Early Onset Ulcerative Colitis
- Two Cases of Refractory Pseudomembranous Colitis that Healed Following Fecal Microbiota Transplantation
- Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
- Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient
- Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis?