Current new challenges in the management of ulcerative colitis
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. takagast@z2.keio.jp
- KMID: 2438441
- DOI: http://doi.org/10.5217/ir.2018.00126
Abstract
- Ulcerative colitis (UC) is a chronic inflammatory condition of the gastrointestinal tract. Although the cause of UC is postulated to be multifactorial in nature, including genetic predisposition, epithelial barrier defects, dysregulation of immune responses, and environmental factors, the specific pathogenesis of UC is still incompletely understood. In the treatment of UC so far, a method of suppressing immunity and treating it has been mainstream. Immunosuppressant drugs, including thiopurines (azathioprine or 6-mercaptopurine), anti-tumor necrosis factor-α (anti-TNF-α) antibody (infliximab and adalimumab), and calcineurin inhibitor, can be used in treat patients with corticosteroid-dependent and/or corticosteroid-refractory moderate-to-severe UC. Recently, in addition to such a conventional therapeutic agent, golimumab, which is the first transgenic human monoclonal anti-TNF-α antibody to be fabricated, anti α-4/β-7 integrin antibody, and Janus kinase inhibitor have been reported to novel immunosuppressant therapy. Furthermore, other treatments with unique mechanisms different from immunosuppression, have also been suggested, including fecal microbiota transplantation and Indigo naturalis, which is a Chinese herbal medicine. We compared the features and efficacy of these new treatments. In this issue, the features and treatment options for these new treatments is reviewed.
Keyword
MeSH Terms
Figure
Cited by 5 articles
-
Efficacy and Safety of Fecal Microbiota Transplantation and Prospect of Microbe-based Therapies for Inflammatory Bowel Disease
Hoon Gil Jo, Geom Seog Seo
Korean J Gastroenterol. 2021;78(1):31-36. doi: 10.4166/kjg.2021.089.Clinical Course of COVID-19 in Patients with Inflammatory Bowel Disease in Korea: a KASID Multicenter Study
Jin Wook Lee, Eun Mi Song, Sung-Ae Jung, Sung Hoon Jung, Kwang Woo Kim, Seong-Joon Koh, Hyun Jung Lee, Seung Wook Hong, Jin Hwa Park, Sung Wook Hwang, Dong-Hoon Yang, Byong Duk Ye, Jeong-Sik Byeon, Seung-Jae Myung, Suk-Kyun Yang, Sang Hyoung Park,
J Korean Med Sci. 2021;36(48):e336. doi: 10.3346/jkms.2021.36.e336.Does cytomegalovirus load predict the outcome of acute severe ulcerative colitis?
You Sun Kim
Intest Res. 2021;19(4):357-359. doi: 10.5217/ir.2021.00120.Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy
Danny Con, Bridgette Andrew, Steven Nicolaides, Daniel R van Langenberg, Abhinav Vasudevan
Intest Res. 2022;20(1):101-113. doi: 10.5217/ir.2020.00146.Reviewing not Homer’s
Iliad , but “Kai Bao Ben Cao ”: indigo dye—the past, present, and future
Yusuke Yoshimatsu, Tomohisa Sujino, Takanori Kanai
Intest Res. 2023;21(2):174-176. doi: 10.5217/ir.2022.00018.
Reference
-
1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article2. Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001; 121:255–260.
Article3. Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003; 2:736–746.4. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article5. Adalimumab in the treatment of moderate-to-severe ulcerative colitis: ULTRA 2 trial results. Gastroenterol Hepatol (N Y). 2013; 9:317–320.6. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:85–95.
Article7. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:96–109. e1.
Article8. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009; 104:760–767.
Article9. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013; 108:40–47.
Article10. Sofia MA, Rubin DT. Current approaches for optimizing the benefit of biologic therapy in ulcerative colitis. Therap Adv Gastroenterol. 2016; 9:548–559.
Article11. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015; 148:1320–1329. e3.
Article12. Cesarini M, Katsanos K, Papamichael K, et al. Dose optimization is effective in ulcerative colitis patients losing response to infliximab: a collaborative multicentre retrospective study. Dig Liver Dis. 2014; 46:135–139.
Article13. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018; 390:2779–2789.
Article14. Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol. 2014; 49:100–109.
Article15. Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol. 2012; 47:136–143.
Article16. Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004; 2:542–553.
Article17. Panccione R, Ghosh S, Middleton S, et al. Infliximab, azathioprine, or infliximab + azathioprine for treatment of moderate to severe ulcerative colitis: the UC success trial. Gastroenterology. 2011; 140:S–134.
Article18. Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016; 15:173–183.
Article19. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article20. Engel T, Ungar B, Yung DE, Ben-Horin S, Eliakim R, Kopylov U. Vedolizumab in IBD-lessons from real-world experience: a systematic review and pooled analysis. J Crohns Colitis. 2018; 12:245–257.
Article21. Kopylov U, Avni-Biron I, Ron Y, et al. Effectiveness and safety of vedolizumab for maintenance treatment in inflammatory bowel disease: the Israeli real world experience. Dig Liver Dis. 2019; 51:68–74.
Article22. Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016; 14:1593–1601. e2.23. Christensen B, Gibson P, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol. [published online ahead of print May 8, 2018].https://doi.org/10.1016/j.cgh.2018.04.060.
Article24. Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017; 11:400–411.
Article25. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014; 57:5023–5038.
Article26. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article27. Choy E, Sattar N. Interpreting lipid levels in the context of highgrade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009; 68:460–469.
Article28. Wu JJ, Strober BE, Hansen PR, et al. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J Am Acad Dermatol. 2016; 75:897–905.
Article29. Vickers AD, Ainsworth C, Mody R, et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One. 2016; 11:e0165435.
Article30. Adachi J, Mori Y, Matsui S, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001; 276:31475–31478.
Article31. Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013; 39:372–385.
Article32. Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013; 39:386–399.
Article33. Kawai S, Iijima H, Shinzaki S, et al. Indigo naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol. 2017; 52:904–919.
Article34. Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016; 51:853–861.
Article35. Lin YK, Wong WR, Chang YC, et al. The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque-type psoriasis. Dermatology. 2007; 214:155–161.
Article36. Deng S, May BH, Zhang AL, Lu C, Xue CC. Plant extracts for the topical management of psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013; 169:769–782.
Article37. Sugimoto S, Naganuma M, Kiyohara H, et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016; 93:193–201.
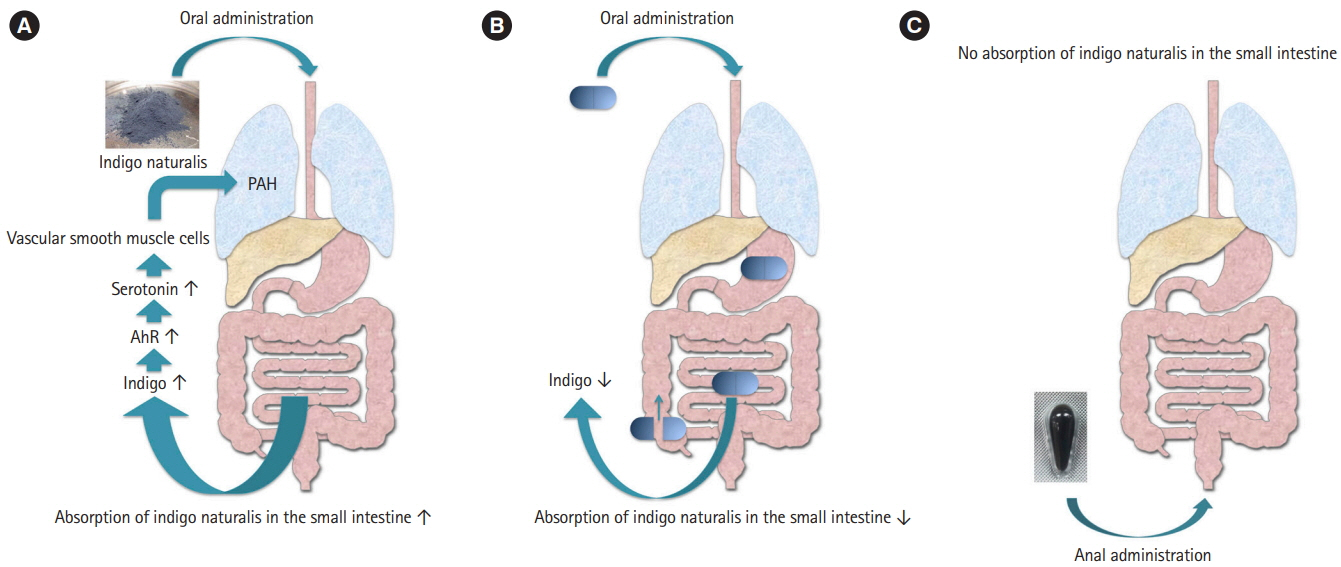
Article38. Naganuma M, Sugimoto S, Mitsuyama K, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018; 154:935–947.39. Nishio M, Hirooka K, Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur Heart J. 2016; 37:1992.
Article40. Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011; 8:443–455.
Article41. Kondo S, Araki T, Okita Y, et al. Colitis with wall thickening and edematous changes during oral administration of the powdered form of Qing-Dai in patients with ulcerative colitis: a report of two cases. Clin J Gastroenterol. 2018; 11:268–272.
Article42. Razik R, Rumman A, Bahreini Z, McGeer A, Nguyen GC. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol. 2016; 111:1141–1146.
Article43. Nagao-Kitamoto H, Kamada N. Host-microbial cross-talk in inflammatory bowel disease. Immune Netw. 2017; 17:1–12.
Article44. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109. e6.
Article45. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–118. e4.
Article46. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017; 389:1218–1228.
Article47. Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017; 46:213–224.
Article