Cancer Res Treat.
2019 Jan;51(1):43-52. 10.4143/crt.2017.562.
Randomized Open Label Phase III Trial of Irinotecan Plus Capecitabine versus Capecitabine Monotherapy in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane: PROCEED Trial (KCSG BR 11-01)
- Affiliations
-
- 1Division of Internal Medicine, Center for Breast Cancer, National Cancer Center, Goyang, Korea. jungsro@ncc.re.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Oncology, Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea.
- 4Department of Oncology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University College of Medicine, Seoul, Korea.
- 6Division of Oncology/Hematology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 7Division of Oncology/Hematology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 8Center for Clinical Trials, National Cancer Center, Goyang, Korea.
- 9Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2437597
- DOI: http://doi.org/10.4143/crt.2017.562
Abstract
- PURPOSE
We investigated whether irinotecan plus capecitabine improved progression-free survival (PFS) compared with capecitabine alone in patients with human epidermal growth factor 2 (HER2) negative and anthracycline and taxane pretreated metastatic breast cancer (MBC).
MATERIALS AND METHODS
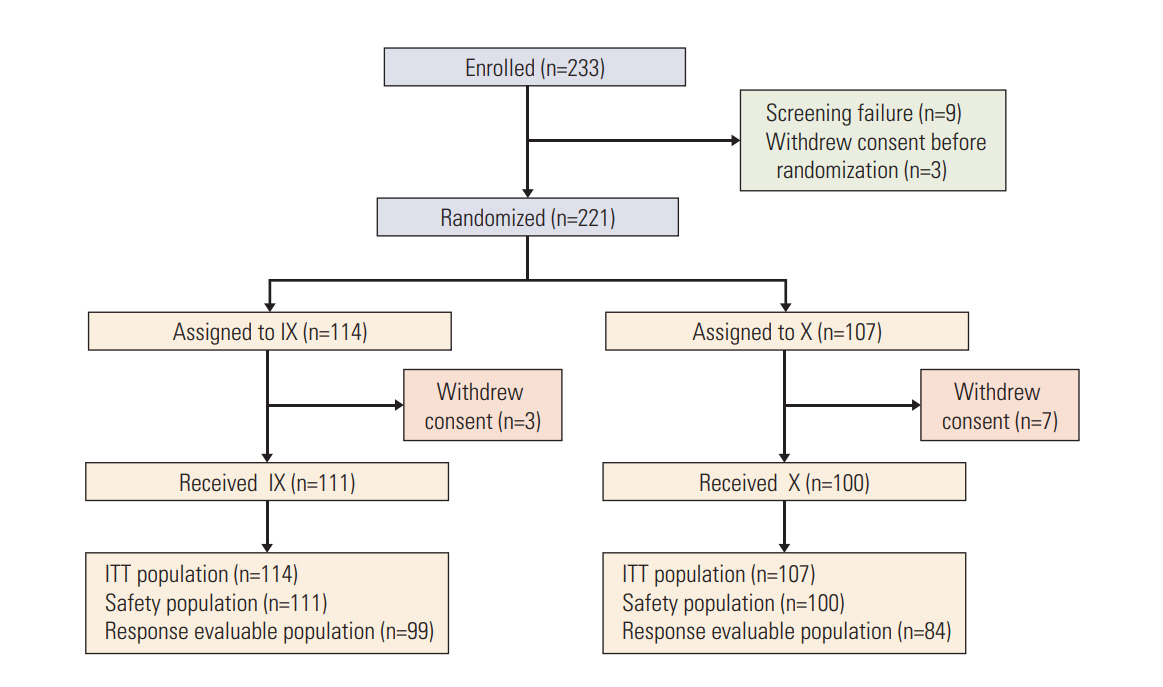
A total of 221 patients were randomly assigned to irinotecan (80 mg/m2, days 1 and 8) and capecitabine (1,000 mg/m2 twice a day, days 1-14) or capecitabine alone (1,250 mg/m2 twice a day, days 1-14) every 3 weeks. The primary endpoint was PFS.
RESULTS
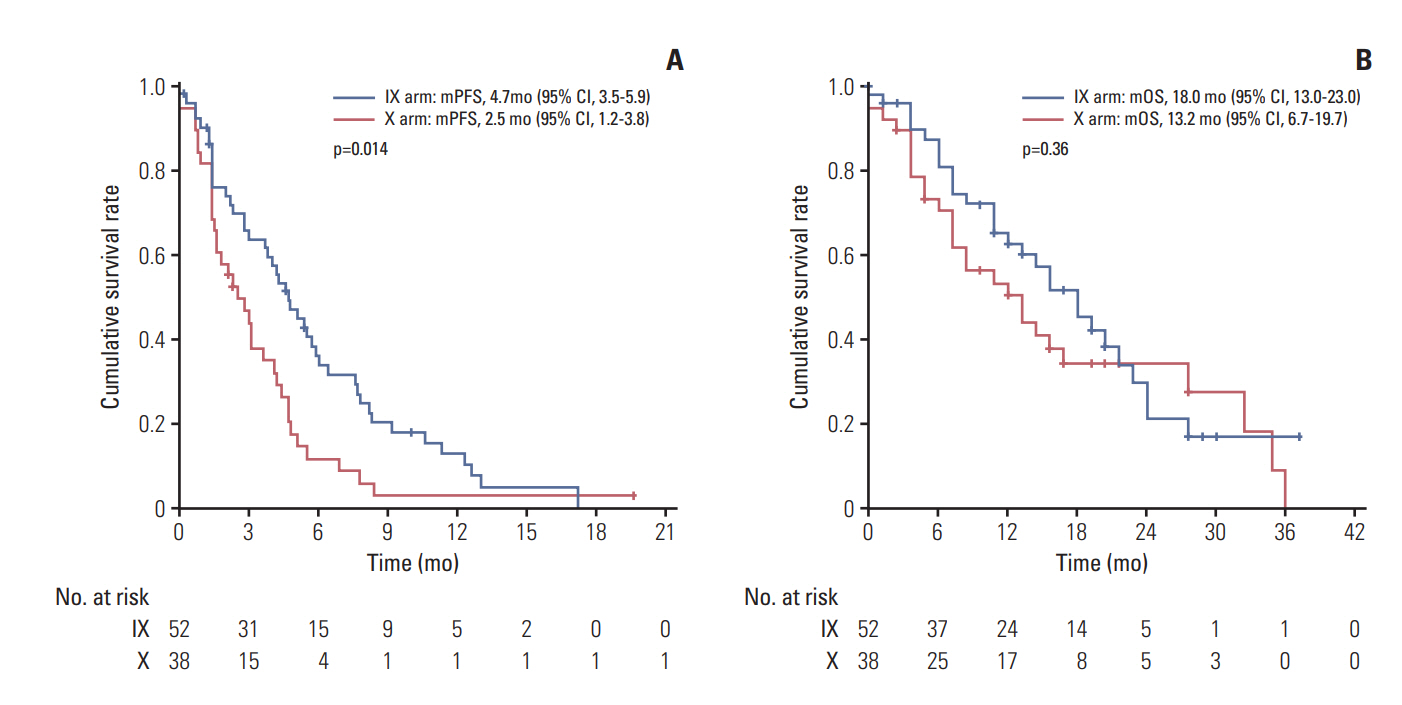
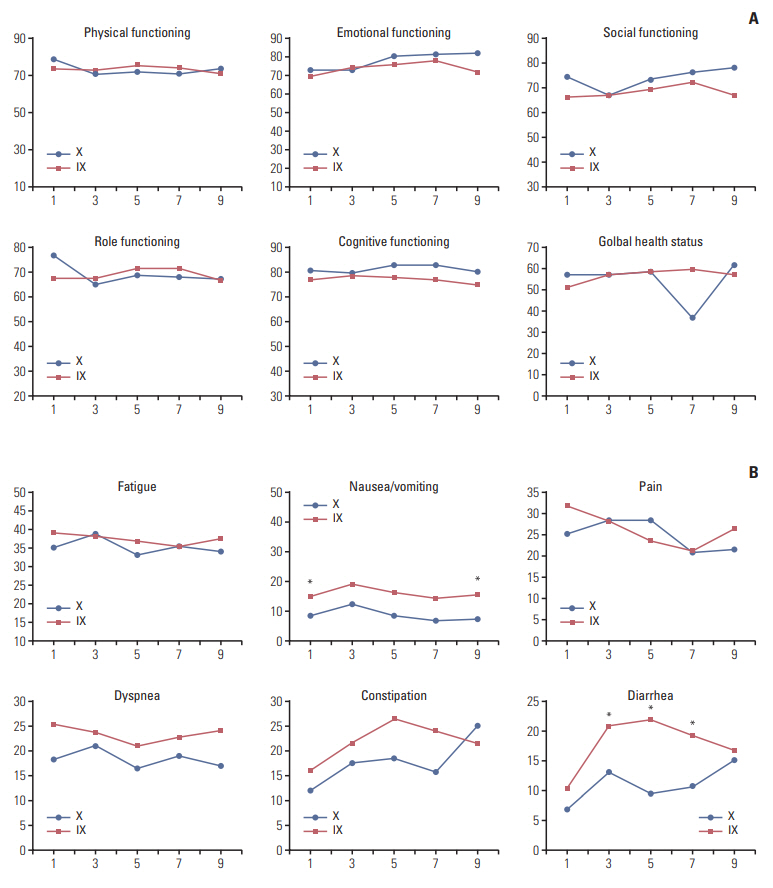
There was no significant difference in PFS between the combination and monotherapy arm (median, 6.4 months vs. 4.7 months; hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.63 to 1.11; p=0.84). In patients with triple-negative breast cancer (TNBC, n=90), the combination significantly improved PFS (median, 4.7 months vs. 2.5 months; HR, 0.58; 95% CI, 0.37 to 0.91; p=0.02). Objective response rate was numerically higher in the combination arm, though it failed to reach statistical significance (44.4% vs. 33.3%, p=0.30). Overall survival did not differ between arms (median, 20.4 months vs. 24.0 months; p=0.63). While grade 3 or 4 neutropenia was more common in the combination arm (39.6% vs. 9.0%), hand-foot syndrome was more often observed in capecitabine arm. Quality of life measurements in global health status was similar. However, patients in the combination arm showed significantly worse symptom scales especially in nausea/vomiting and diarrhea.
CONCLUSION
Irinotecan plus capecitabine did not prove clinically superior to single-agent capecitabine in anthracycline- and taxane-pretreated HER2 negative MBC patients. Toxicity profiles of the two groups differed but were manageable. The role of added irinotecan in patients with TNBC remains to be elucidated.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Ann Oncol. 2014; 25:1871–88.2. Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002; 3:430–40.
Article3. Pizzolato JF, Saltz LB. The camptothecins. Lancet. 2003; 361:2235–42.
Article4. Ichikawa W, Takahashi T, Suto K, Yamashita T, Nihei Z, Shirota Y, et al. Thymidylate synthase predictive power is overcome by irinotecan combination therapy with S-1 for gastric cancer. Br J Cancer. 2004; 91:1245–50.
Article5. Guo Y, Shi M, Shen X, Yang C, Yang L, Zhang J. Capecitabine plus irinotecan versus 5-FU/leucovorin plus irinotecan in the treatment of colorectal cancer: a meta-analysis. Clin Colorectal Cancer. 2014; 13:110–8.
Article6. O'Connor T, Rustum Y, Levine E, Creaven P. A phase I study of capecitabine and a modulatory dose of irinotecan in metastatic breast cancer. Cancer Chemother Pharmacol. 2008; 61:125–31.7. Perez EA, Hillman DW, Mailliard JA, Ingle JN, Ryan JM, Fitch TR, et al. Randomized phase II study of two irinotecan schedules for patients with metastatic breast cancer refractory to an anthracycline, a taxane, or both. J Clin Oncol. 2004; 22:2849–55.
Article8. Tan WW, Hillman DW, Salim M, Northfelt DW, Anderson DM, Stella PJ, et al. N0332 phase 2 trial of weekly irinotecan hydrochloride and docetaxel in refractory metastatic breast cancer: a North Central Cancer Treatment Group (NCCTG) Trial. Ann Oncol. 2010; 21:493–7.
Article9. Stathopoulos GP, Tsavdaridis D, Malamos NA, Rigatos SK, Kosmas C, Pergantas N, et al. Irinotecan combined with docetaxel in pre-treated metastatic breast cancer patients: a phase II study. Cancer Chemother Pharmacol. 2005; 56:487–91.
Article10. Lee KS, Park IH, Nam BH, Ro J. Phase II study of irinotecan plus capecitabine in anthracycline- and taxane- pretreated patients with metastatic breast cancer. Invest New Drugs. 2013; 31:152–9.
Article11. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85:365–76.
Article12. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004; 13:863–8.
Article13. Jassem J, Carroll C, Ward SE, Simpson E, Hind D. The clinical efficacy of cytotoxic agents in locally advanced or metastatic breast cancer patients pretreated with an anthracycline and a taxane: a systematic review. Eur J Cancer. 2009; 45:2749–58.
Article14. Gelmon K, Chan A, Harbeck N. The role of capecitabine in first-line treatment for patients with metastatic breast cancer. Oncologist. 2006; 11 Suppl 1:42–51.
Article15. Belfiglio M, Fanizza C, Tinari N, Ficorella C, Iacobelli S, Natoli C, et al. Meta-analysis of phase III trials of docetaxel alone or in combination with chemotherapy in metastatic breast cancer. J Cancer Res Clin Oncol. 2012; 138:221–9.
Article16. O'Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002; 20:2812–23.17. Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan JP, Llombart AC, Pluzanska A, et al. Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008; 26:3950–7.
Article18. Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010; 28:3256–63.
Article19. Perez EA, Awada A, O'Shaughnessy J, Rugo HS, Twelves C, Im SA, et al. Etirinotecan pegol (NKTR-102) versus treatment of physician's choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015; 16:1556–68.
Article20. Hoch U, Staschen CM, Johnson RK, Eldon MA. Nonclinical pharmacokinetics and activity of etirinotecan pegol (NKTR-102), a long-acting topoisomerase 1 inhibitor, in multiple cancer models. Cancer Chemother Pharmacol. 2014; 74:1125–37.
Article21. Twelves C, Cortes J, O'Shaughnessy J, Awada A, Perez EA, Im SA, et al. Health-related quality of life in patients with locally recurrent or metastatic breast cancer treated with etirinotecan pegol versus treatment of physician's choice: results from the randomised phase III BEACON trial. Eur J Cancer. 2017; 76:205–15.
Article22. Cortes J, Rugo HS, Awada A, Twelves C, Perez EA, Im SA, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017; 165:329–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Capecitabine Monotherapy in Taxane-Refractory Metastatic Breast Cancer (MBC) Patients
- Gemcitabine Single or Combination Chemotherapy in Post Anthracycline and Taxane Salvage Treatment of Metastatic Breast Cancer: Retrospective Analysis of 124 Patients
- Capecitabine and Vinorelbine in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane
- A Phase II Study of Gemcitabine Monotherapy in Breast Cancer Patients Refractory to Anthracycline and Taxane
- Chemotherapy for Colorecal Cancer