J Pathol Transl Med.
2019 Jan;53(1):40-49. 10.4132/jptm.2018.11.29.
Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. junghokim@snuh.org, ghkang@snu.ac.kr
- 2Laboratory of Epigenetics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2437577
- DOI: http://doi.org/10.4132/jptm.2018.11.29
Abstract
- BACKGROUND
This study aimed to investigate the prognostic impact of intratumoral Fusobacterium nucleatum in colorectal cancer (CRC) treated with adjuvant chemotherapy.
METHODS
F. nucleatum DNA was quantitatively measured in a total of 593 CRC tissues retrospectively collected from surgically resected specimens of stage III or high-risk stage II CRC patients who had received curative surgery and subsequent oxaliplatin-based adjuvant chemotherapy (either FOLFOXor CAPOX). Each case was classified into one of the three categories: F. nucleatum-high, -low, or -negative.
RESULTS
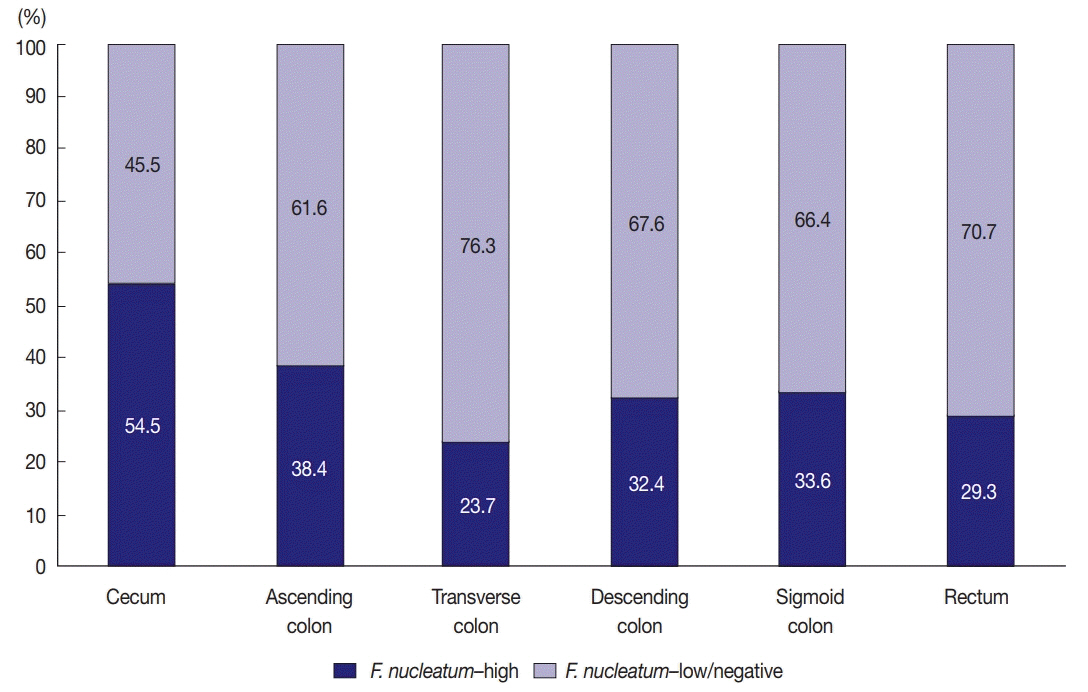
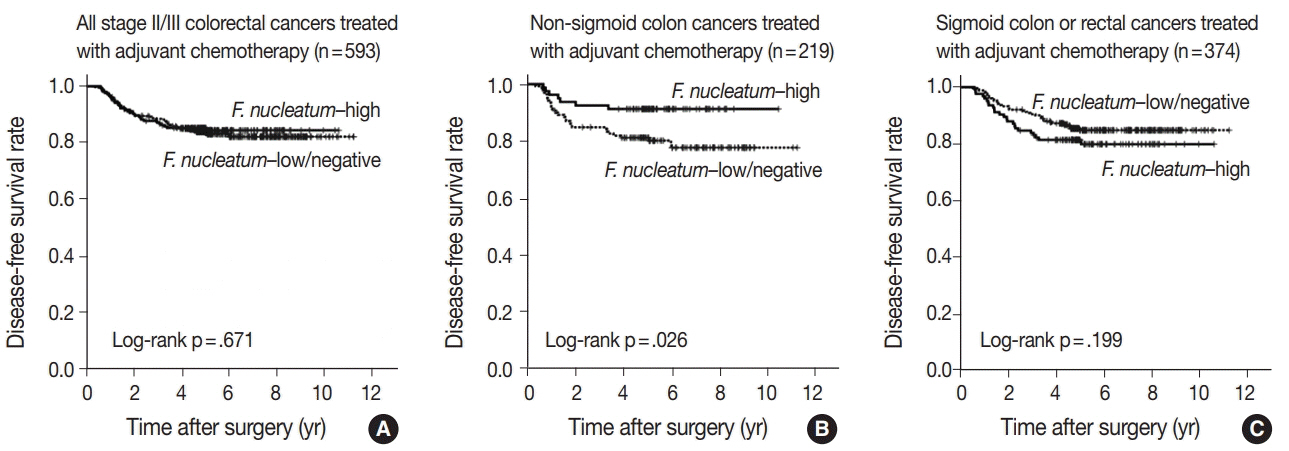
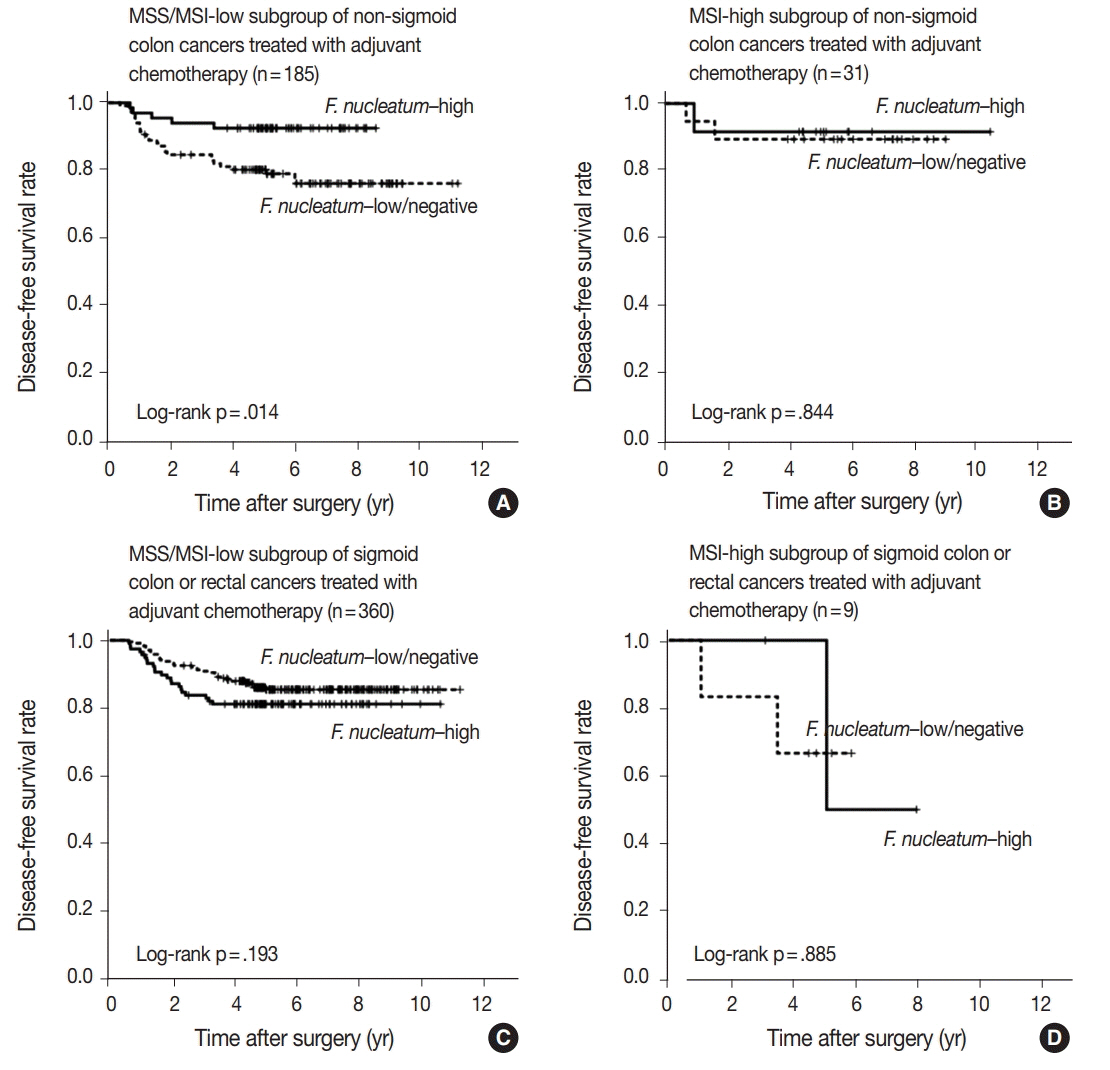
No significant differences in survival were observed between the F.nucleatum-high and -low/negative groups in the 593 CRCs (p = .671). Subgroup analyses according to tumor location demonstrated that disease-free survival was significantly better in F.nucleatum-high than in -low/negative patients with non-sigmoid colon cancer (including cecal, ascending, transverse, and descending colon cancers; n = 219; log-rank p = .026). In multivariate analysis, F. nucleatum was determined to be an independent prognostic factor in non-sigmoid colon cancers (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.97; p = .043). Furthermore, the favorable prognostic effect of F. nucleatum-high was observed only in a non-microsatellite instability-high (non-MSI-high) subset of non-sigmoid colon cancers (log-rank p = 0.014), but not in a MSI-high subset (log-rank p = 0.844), suggesting that the combined status of tumor location and MSI may be a critical factor for different prognostic impacts of F. nucleatum in CRCs treated with adjuvant chemotherapy.
CONCLUSIONS
Intratumoral F. nucleatum load is a potential prognostic factor in a non-MSI-high/non-sigmoid/non-rectal cancer subset of stage II/III CRCs treated with oxaliplatin-based adjuvant chemotherapy.
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic and clinicopathological significance of
Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
J Pathol Transl Med. 2022;56(3):144-151. doi: 10.4132/jptm.2022.03.13.
Reference
-
1. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumorimmune microenvironment. Cell Host Microbe. 2013; 14:207–15.
Article2. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013; 14:195–206.3. Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016; 65:1973–80.4. Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017; 170:548–63. e16.
Article5. Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017; 357:1156–60.
Article6. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012; 22:292–8.
Article7. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012; 22:299–306.
Article8. Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014; 74:1311–8.
Article9. Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015; 137:1258–68.10. Yu J, Chen Y, Fu X, et al. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016; 139:1318–26.11. Flanagan L, Schmid J, Ebert M, et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis. 2014; 33:1381–90.
Article12. Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015; 1:653–61.13. Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Transl Gastroenterol. 2016; 7:e200.
Article14. Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017; 471:329–36.
Article15. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018; 359:91–7.16. Vetizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015; 350:1079–84.
Article17. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018; 359:97–103.18. Bae JM, Kim JH, Oh HJ, et al. Downregulation of acetyl-CoA synthetase 2 is a metabolic hallmark of tumor progression and aggressiveness in colorectal carcinoma. Mod Pathol. 2017; 30:267–77.
Article19. Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58:5248–57.20. Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2018; 53:517–24.
Article21. Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017; 84:69–80.
Article22. Bae JM, Kim JH, Kang GH. Molecular subtypes of colorectal cancer and their clinicopathologic features, with an emphasis on the serrated neoplasia pathway. Arch Pathol Lab Med. 2016; 140:406–12.
Article23. Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014; 111:18321–6.
Article24. Abed J, Emgård JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumorexpressed Gal-GalNAc. Cell Host Microbe. 2016; 20:215–25.
Article25. Hamada T, Zhang X, Mima K, et al. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res. 2018; 6:1327–36.
Article26. Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018; 391:2128–39.27. Lee DW, Han SW, Kang JK, et al. Association between Fusobacterium nucleatum, pathway mutation, and patient prognosis in colorectal cancer. Ann Surg Oncol. 2018; 25:3389–95.
Article28. Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014; 20:4230–43.
Article29. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018; 10:71–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic Factors Associated with Prognosis after Adjuvant Chemotherapy in Stage II/III Microsatellite-Unstable Colorectal Cancers
- Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
- Genomic Instability in Colorectal Cancer; from Bench to Bed
- Clinicopathological Significance of Microsatellite Instability in Sporadic Colorectal Cancer
- Prognostic Impact of Microsatellite Instability in Colorectal Cancer Presenting With Mucinous, Signet-Ring, and Poorly Differentiated Cells