J Pathol Transl Med.
2015 Mar;49(2):118-128. 10.4132/jptm.2015.02.05.
Pathologic Factors Associated with Prognosis after Adjuvant Chemotherapy in Stage II/III Microsatellite-Unstable Colorectal Cancers
- Affiliations
-
- 1Department of Pathology, SMG-SNU Boramae Medical Center, Seoul, Korea. ghkang@snu.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2381366
- DOI: http://doi.org/10.4132/jptm.2015.02.05
Abstract
- BACKGROUND
Although there are controversies regarding the benefit of fluoropyrimidine-based adjuvant chemotherapy in patients with microsatellite instability-high (MSI-H) colorectal cancer (CRC), the pathologic features affecting postchemotherapeutic prognosis in these patients have not been fully identified yet.
METHODS
A total of 26 histopathologic and immunohistochemical factors were comprehensively evaluated in 125 stage II or III MSI-H CRC patients who underwent curative resection followed by fluoropyrimidine-based adjuvant chemotherapy. We statistically analyzed the associations of these factors with disease-free survival (DFS).
RESULTS
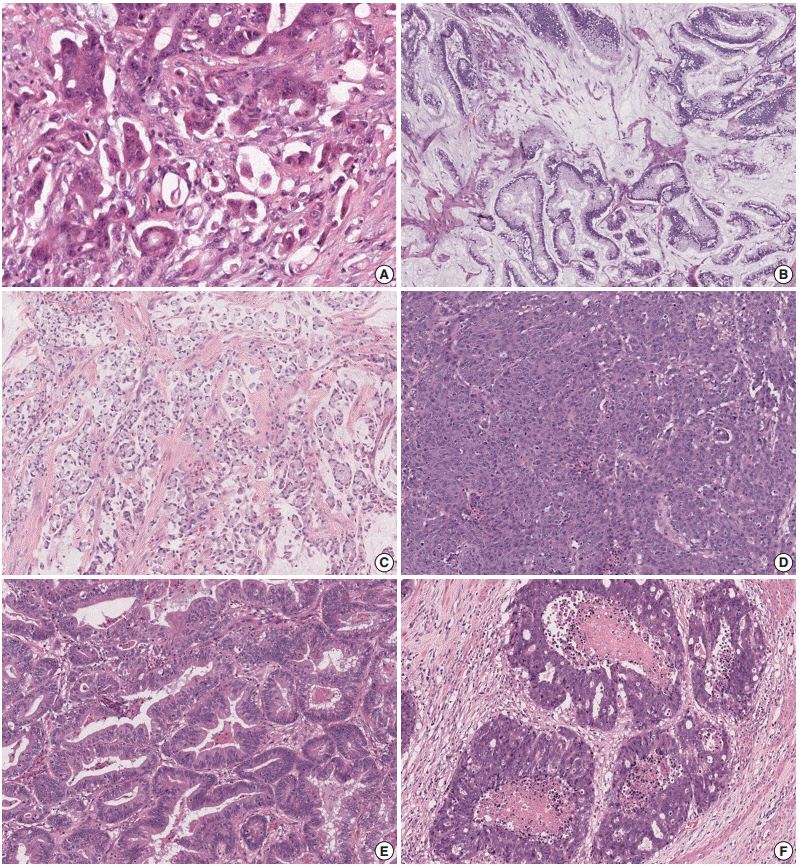
Using a Kaplan- Meier analysis with log-rank test, we determined that ulceroinfiltrative gross type (p=.003), pT4 (p<.001), pN2 (p=.002), perineural invasion (p=.001), absence of peritumoral lymphoid reaction (p=.041), signet ring cell component (p=.006), and cribriform comedo component (p=.004) were significantly associated with worse DFS in patients receiving oxaliplatin-based adjuvant chemotherapy (n=45). By contrast, pT4 (p<.001) and tumor budding-positivity (p=.032) were significant predictors of poor survival in patients receiving non-oxaliplatin-based adjuvant chemotherapy (n=80). In Cox proportional hazards regression model-based univariate and multivariate analyses, pT category (pT1-3 vs pT4) was the only significant prognostic factor in patients receiving non-oxaliplatin-based adjuvant chemotherapy, whereas pT category, signet ring cell histology and cribriform comedo histology remained independent prognostic factors in patients receiving oxaliplatin-based adjuvant chemotherapy.
CONCLUSIONS
pT4 status is the most significant pathologic determinant of poor outcome after fluoropyrimidine-based adjuvant chemotherapy in patients with stage II/III MSI-H CRC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic Predictability of American Joint Committee on Cancer 8th Staging System for Perihilar Cholangiocarcinoma: Limited Improvement Compared with the 7th Staging System
Jong Woo Lee, Jae Hoon Lee, Yejong Park, Woohyung Lee, Jaewoo Kwon, Ki Byung Song, Dae Wook Hwang, Song Cheol Kim
Cancer Res Treat. 2020;52(3):886-895. doi: 10.4143/crt.2020.023.
Reference
-
1. Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008; 134:1296–310.
Article2. Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014; 20:4230–43.
Article3. Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999; 117:123–31.4. Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003; 106:66–73.
Article5. Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007; 133:48–56.
Article6. Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet. 2009; 41:112–7.
Article7. Kim JH, Bae JM, Kim KJ, et al. Differential features of microsatelliteunstable colorectal carcinomas depending on EPCAM expression status. Korean J Pathol. 2014; 48:276–82.
Article8. Kim JH, Rhee YY, Bae JM, Cho NY, Kang GH. Loss of CDX2/CK20 expression is associated with poorly differentiated carcinoma, the CpG island methylator phenotype, and adverse prognosis in microsatellite-unstable colorectal cancer. Am J Surg Pathol. 2013; 37:1532–41.
Article9. Ricciardiello L, Ceccarelli C, Angiolini G, et al. High thymidylate synthase expression in colorectal cancer with microsatellite instability: implications for chemotherapeutic strategies. Clin Cancer Res. 2005; 11:4234–40.
Article10. Sinicrope FA, Rego RL, Halling KC, et al. Thymidylate synthase expression in colon carcinomas with microsatellite instability. Clin Cancer Res. 2006; 12:2738–44.
Article11. Popat S, Wort R, Houlston RS. Inter-relationship between microsatellite instability, thymidylate synthase expression, and p53 status in colorectal cancer: implications for chemoresistance. BMC Cancer. 2006; 6:150.
Article12. Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007; 25:767–72.
Article13. Dorard C, de Thonel A, Collura A, et al. Expression of a mutant HSP110 sensitizes colorectal cancer cells to chemotherapy and improves disease prognosis. Nat Med. 2011; 17:1283–9.
Article14. Kim JH, Kim KJ, Rhee YY, et al. Expression status of wild-type HSP110 correlates with HSP110 T17 deletion size and patient prognosis in microsatellite-unstable colorectal cancer. Mod Pathol. 2014; 27:443–53.
Article15. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010.16. Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004; 127:385–94.
Article17. Klintrup K, Mäkinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005; 41:2645–54.
Article18. Ueno H, Hashiguchi Y, Shimazaki H, et al. Objective criteria for crohn-like lymphoid reaction in colorectal cancer. Am J Clin Pathol. 2013; 139:434–41.
Article19. Wick MR, Vitsky JL, Ritter JH, Swanson PE, Mills SE. Sporadic medullary carcinoma of the colon: a clinicopathologic comparison with nonhereditary poorly differentiated enteric-type adenocarcinoma and neuroendocrine colorectal carcinoma. Am J Clin Pathol. 2005; 123:56–65.20. Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013; 62:367–86.
Article21. Chirieac LR, Shen L, Catalano PJ, Issa JP, Hamilton SR. Phenotype of microsatellite-stable colorectal carcinomas with CpG island methylation. Am J Surg Pathol. 2005; 29:429–36.
Article22. Johnston PG, Fisher ER, Rockette HE, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol. 1994; 12:2640–7.
Article23. Marzouk O, Schofield J. Review of histopathological and molecular prognostic features in colorectal cancer. Cancers (Basel). 2011; 3:2767–810.
Article24. Richards CH, Flegg KM, Roxburgh CS, et al. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. 2012; 106:2010–5.
Article25. Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010; 29:482–91.
Article26. Ross JS, Torres-Mora J, Wagle N, Jennings TA, Jones DM. Biomarker-based prediction of response to therapy for colorectal cancer: current perspective. Am J Clin Pathol. 2010; 134:478–90.27. Li J, Guo BC, Sun LR, et al. TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J Gastroenterol. 2014; 20:5104–12.
Article28. Snaebjornsson P, Coupe VM, Jonasson L, Meijer GA, van Grieken NC, Jonasson JG. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis: Shepherd’s local peritoneal involvement revisited. Int J Cancer. 2014; 135:467–78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy
- Is Microsatellite Instability Really a Good Prognostic Factor of Colorectal Cancer?
- Adjuvant chemotherapy for patients with stage II high-risk and III colon cancer: Hindering factors to adherence and impact on long-term survival
- Clinicopathological Significance of Microsatellite Instability in Sporadic Colorectal Cancer
- Genomic Instability in Colorectal Cancer; from Bench to Bed