Clin Exp Otorhinolaryngol.
2019 Feb;12(1):33-39. 10.21053/ceo.2018.00542.
Losartan Prevents Maladaptive Auditory-Somatosensory Plasticity After Hearing Loss via Transforming Growth Factor-β Signaling Suppression
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chung-Ang University College of Medicine, Seoul, Korea. caduent@gmail.com
- 2Department of Otorhinolaryngology, National Medical Center, Seoul, Korea.
- 3Department of Neurology, Chung-Ang University College of Medicine, Seoul, Korea.
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Veterans Health Service Medical Center, Seoul, Korea.
- 5Biomedical Research Institute, Chung-Ang University Hospital, Seoul, Korea.
- KMID: 2437489
- DOI: http://doi.org/10.21053/ceo.2018.00542
Abstract
OBJECTIVES
Hearing loss disrupts the balance of auditory-somatosensory inputs in the cochlear nucleus (CN) of the brainstem, which has been suggested to be a mechanism of tinnitus. This disruption results from maladaptive auditory-somatosensory plasticity, which is a form of axonal sprouting. Axonal sprouting is promoted by transforming growth factor (TGF)-β signaling, which can be inhibited by losartan. We investigated whether losartan prevents maladaptive auditory-somatosensory plasticity after hearing loss.
METHODS
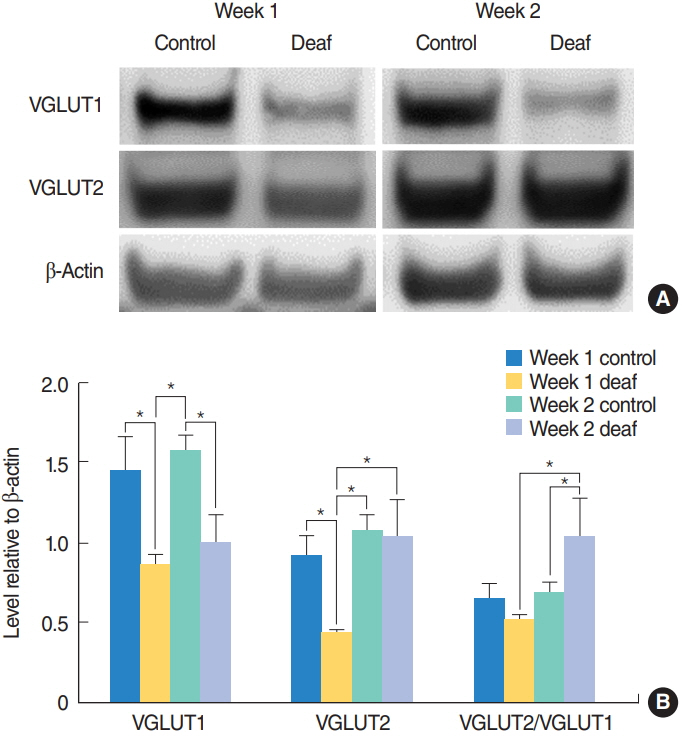
The study consisted of two stages: determining the time course of auditory-somatosensory plasticity following hearing loss and preventing auditory-somatosensory plasticity using losartan. In the first stage, rats were randomly divided into two groups: a control group that underwent a sham operation and a deaf group that underwent cochlea ablation on the left side. CNs were harvested 1 and 2 weeks after surgery. In the second stage, rats were randomly divided into either a saline group that underwent cochlear ablation on the left side and received normal saline or a losartan group that underwent cochlear ablation on the left side and received losartan. CNs were harvested 2 weeks after surgery. Hearing was estimated with auditory brainstem responses (ABRs). Western blotting was performed for vesicular glutamate transporter 1 (VGLUT1), reflecting auditory input; vesicular glutamate transporter 2 (VGLUT2), reflecting somatosensory input; growth-associated protein 43 (GAP-43), reflecting axonal sprouting; and p-Smad2/3.
RESULTS
Baseline ABR thresholds before surgery ranged from 20 to 35 dB sound pressure level. After cochlear ablation, ABR thresholds were higher than 80 dB. In the first experiment, VGLUT2/VGLUT1 ratios did not differ significantly between the control and deaf groups 1 week after surgery. At 2 weeks after surgery, the deaf group had a significantly higher VGLUT2/VGLUT1 ratio compared to the control group. In the second experiment, the losartan group had a significantly lower VGLUT2/VGLUT1 ratio along with significantly lower p-Smad3 and GAP-43 levels compared to the saline group.
CONCLUSION
Losartan might prevent axonal sprouting after hearing loss by blocking TGF-β signaling thereby preventing maladaptive auditory-somatosensory plasticity.
MeSH Terms
-
Animals
Axons
Blotting, Western
Brain Stem
Cochlea
Cochlear Nucleus
Evoked Potentials, Auditory, Brain Stem
GAP-43 Protein
Hearing Loss*
Hearing*
Losartan*
Plastics*
Rats
Tinnitus
Transforming Growth Factors
Vesicular Glutamate Transport Protein 1
Vesicular Glutamate Transport Protein 2
GAP-43 Protein
Losartan
Plastics
Transforming Growth Factors
Vesicular Glutamate Transport Protein 1
Vesicular Glutamate Transport Protein 2
Figure
Cited by 1 articles
-
MicroRNAs Related to Cognitive Impairment After Hearing Loss
Seog-Kyun Mun, Hyunkyu Chae, Xian-Yu Piao, Hyun-Jin Lee, Young-Kook Kim, Seung-Ha Oh, Munyoung Chang
Clin Exp Otorhinolaryngol. 2021;14(1):76-81. doi: 10.21053/ceo.2019.01382.
Reference
-
1. Wu C, Stefanescu RA, Martel DT, Shore SE. Tinnitus: maladaptive auditory-somatosensory plasticity. Hear Res. 2016; Apr. 334:20–9.
Article2. Zhan X, Pongstaporn T, Ryugo DK. Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol. 2006; May. 496(3):335–48.
Article3. Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006; Mar. 495(1):100–12.
Article4. Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus: possible basis for tinnitus-related hyperactivity. J Neurosci. 2012; Feb. 32(5):1660–71.5. Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013; Dec. 33(50):19647–56.
Article6. Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009; Apr. 29(13):4210–7.
Article7. Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci. 2012; Nov. 32(45):15791–801.
Article8. Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007; Feb. 500(4):777–87.
Article9. Zeng C, Shroff H, Shore SE. Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2. Neuroscience. 2011; Mar. 176:142–51.
Article10. Boulland JL, Ferhat L, Tallak Solbu T, Ferrand N, Chaudhry FA, Storm-Mathisen J, et al. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol. 2007; Jul. 503(3):466–85.11. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Burlington (MA): Academic Press;2006.12. Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One. 2013; 8(3):e59828.
Article13. Bilak M, Kim J, Potashner SJ, Bohne BA, Morest DK. New growth of axons in the cochlear nucleus of adult chinchillas after acoustic trauma. Exp Neurol. 1997; Oct. 147(2):256–68.
Article14. Fuentes-Santamaria V, Alvarado JC, Henkel CK, Brunso-Bechtold JK. Cochlear ablation in adult ferrets results in changes in insulin-like growth factor-1 and synaptophysin immunostaining in the cochlear nucleus. Neuroscience. 2007; Sep. 148(4):1033–47.
Article15. Illing RB, Horvath M. Re-emergence of GAP-43 in cochlear nucleus and superior olive following cochlear ablation in the rat. Neurosci Lett. 1995; Jul. 194(1-2):9–12.
Article16. Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007; Feb. 13(2):204–10.17. Bar-Klein G, Cacheaux LP, Kamintsky L, Prager O, Weissberg I, Schoknecht K, et al. Losartan prevents acquired epilepsy via TGF-β signaling suppression. Ann Neurol. 2014; Jun. 75(6):864–75.
Article18. Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, et al. GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci. 2015; Dec. 18(12):1737–45.
Article19. Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997; Feb. 20(2):84–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atoh1 as a Coordinator of Sensory Hair Cell Development and Regeneration in the Cochlea
- The Role of Tripartite Motif Family Proteins in TGF-β Signaling Pathway and Cancer
- Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint
- Transforming Growth Factor β Receptor Type I Inhibitor, Galunisertib, Has No Beneficial Effects on Aneurysmal Pathological Changes in Marfan Mice
- Targeting the Transforming Growth Factor-beta Signaling in Cancer Therapy