Intest Res.
2018 Oct;16(4):554-562. 10.5217/ir.2018.00027.
Usefulness of fecal calprotectin by monoclonal antibody testing in adult Japanese with inflammatory bowel diseases: a prospective multicenter study
- Affiliations
-
- 1Division of Internal Medicine, Department of Inflammatory Bowel Disease, Hyogo College of Medicine, Nishinomiya, Japan. ibd-im@hyo-med.ac.jp
- 2Department of Medicine, Shiga University of Medical Science, Otsu, Japan.
- 3Center for Clinical Research and Education, Hyogo College of Medicine, Nishinomiya, Japan.
- 4Department of Gastroenterology and Hepatology, Graduate School of Medicine Kyoto University, Kyoto, Japan.
- 5Division of Gastroenterology and Hepatology, Amagasaki Central Hospital, Amagasaki, Japan.
- 6Oku Clinic, Higashiosaka, Japan.
- 7Department of Gastroenterology, Osaka City University Graduate School of Medicine, Osaka, Japan.
- 8Sanyo Chemical Industries, Ltd., Kyoto, Japan.
- 9Department of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Sapporo, Japan.
- KMID: 2434157
- DOI: http://doi.org/10.5217/ir.2018.00027
Abstract
- BACKGROUND/AIMS
Noninvasive objective monitoring is advantageous for optimizing treatment strategies in patients inflammatory bowel disease (IBD). Fecal calprotectin (FCP) is superior to traditional biomarkers in terms of assessing the activity in patients with IBD. However, there are the differences among several FCP assays in the dynamics of FCP. In this prospective multicenter trial, we investigated the usefulness of FCP measurements in adult Japanese patients with IBD by reliable enzyme immunoassay using a monoclonal antibody.
METHODS
We assessed the relationship between FCP levels and disease or endoscopic activity in patients with ulcerative colitis (UC, n=64) or Crohn's disease (CD, n=46) compared with healthy controls (HCs, n=64).
RESULTS
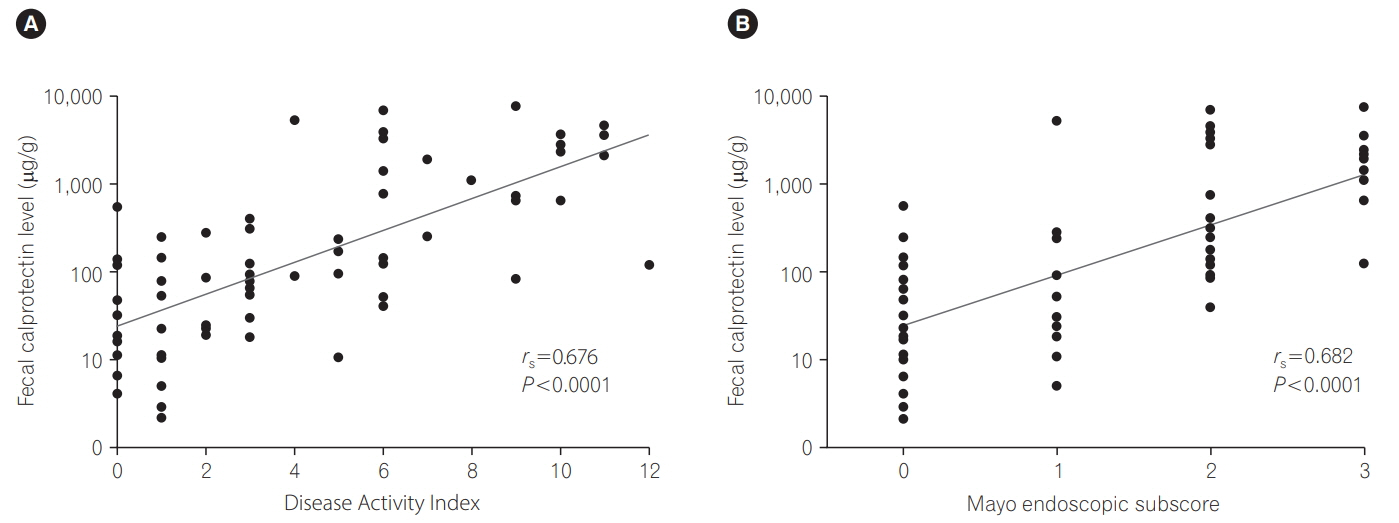
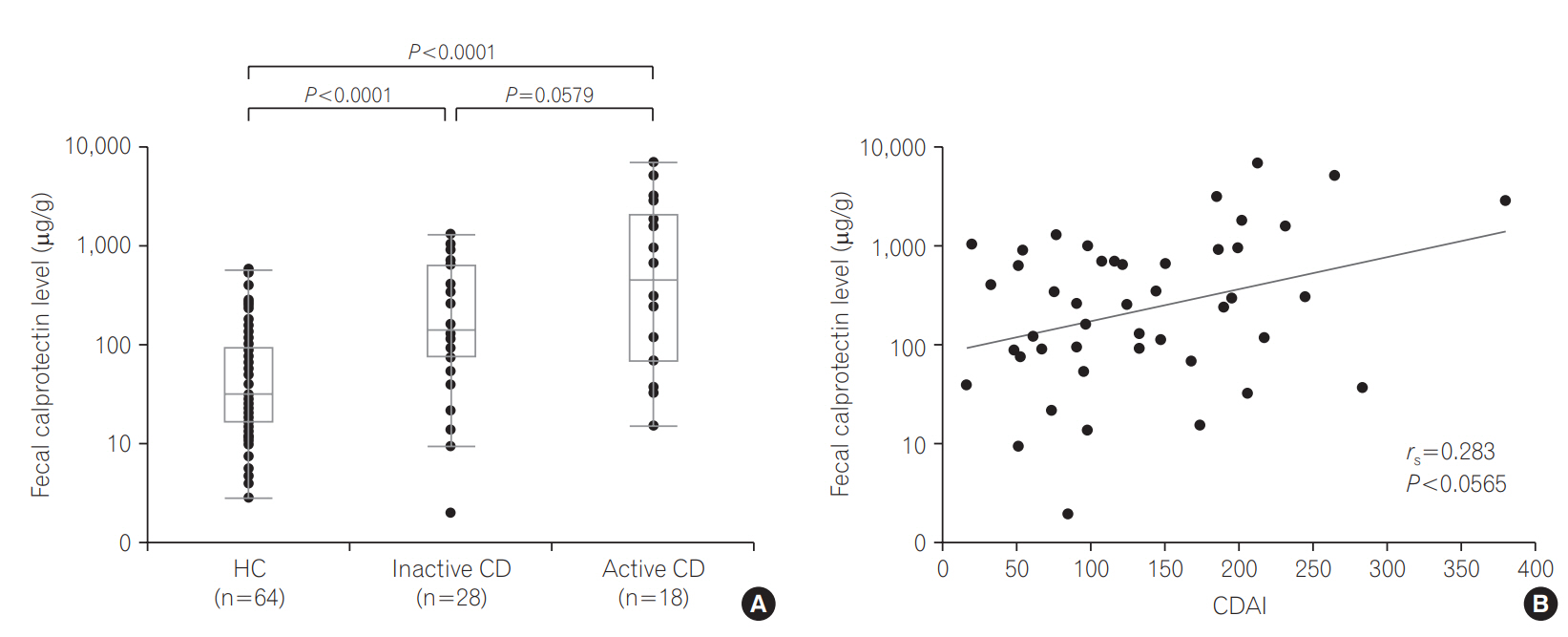
FCP levels in UC patients strongly correlated with the Disease Activity Index (rs =0.676, P < 0.0001) and Mayo endoscopic subscore (MES; rs =0.677, P < 0.0001). FCP levels were significantly higher even in patients with inactive UC or CD compared with HCs (P=0.0068, P < 0.0001). The optimal cutoff value between MES 1 and 2 exhibited higher sensitivity (94.1%). FCP levels were significantly higher in active UC patients than in inactive patients (P < 0.001), except those with proctitis. The Crohn's Disease Activity Index tended to correlate with the FCP level (rs =0.283, P=0.0565).
CONCLUSIONS
Our testing method using a monoclonal antibody for FCP was well-validated and differentiated IBD patients from HCs. FCP may be a useful biomarker for objective assessment of disease activity in adult Japanese IBD patients, especially those with UC.
Keyword
MeSH Terms
-
Adult*
Antibodies, Monoclonal
Asian Continental Ancestry Group*
Biomarkers
Colitis, Ulcerative
Crohn Disease
Humans
Immunoenzyme Techniques
Inflammatory Bowel Diseases*
Leukocyte L1 Antigen Complex*
Methods
Multicenter Studies as Topic
Proctitis
Prospective Studies*
Antibodies, Monoclonal
Biomarkers
Leukocyte L1 Antigen Complex
Figure
Cited by 1 articles
-
Clinical management for small bowel of Crohn’s disease in the treat-to-target era: now is the time to optimize treatment based on the dominant lesion
Kenji Watanabe
Intest Res. 2020;18(4):347-354. doi: 10.5217/ir.2020.00032.
Reference
-
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017; 389:1741–1755.
Article2. Loftus EV Jr. Crohn’s disease: why the disparity in mortality? Gut. 2006; 55:447–449.
Article3. Selinger CP, Leong RW. Mortality from inflammatory bowel diseases. Inflamm Bowel Dis. 2012; 18:1566–1572.
Article4. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140:1785–1794.
Article5. Ueno F, Matsui T, Matsumoto T, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013; 48:31–72.
Article6. Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014; 89:1553–1563.7. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016; 13:567–579.
Article8. Ket SN, Palmer R, Travis S. Endoscopic disease activity in inflammatory bowel disease. Curr Gastroenterol Rep. 2015; 17:50. doi: 10.1007/s11894-015-0470-0.
Article9. Menees S, Higgins P, Korsnes S, Elta G. Does colonoscopy cause increased ulcerative colitis symptoms? Inflamm Bowel Dis. 2007; 13:12–18.
Article10. Däbritz J, Musci J, Foell D. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J Gastroenterol. 2014; 20:363–375.
Article11. Panes J, Jairath V, Levesque BG. Advances in use of endoscopy, radiology, and biomarkers to monitor inflammatory bowel diseases. Gastroenterology. 2017; 152:362–373. e3.
Article12. Lopez RN, Leach ST, Lemberg DA, Duvoisin G, Gearry RB, Day AS. Fecal biomarkers in inflammatory bowel disease. J Gastroenterol Hepatol. 2017; 32:577–582.
Article13. Manceau H, Chicha-Cattoir V, Puy H, Peoc’h K. Fecal calprotectin in inflammatory bowel diseases: update and perspectives. Clin Chem Lab Med. 2017; 55:474–483.
Article14. Kopylov U, Yung DE, Engel T, et al. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016; 28:1137–1144.
Article15. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:802–819.
Article16. Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014; 109:637–645.
Article17. Ma C, Lumb R, Walker EV, et al. Noninvasive fecal immunochemical testing and fecal calprotectin predict mucosal healing in inflammatory bowel disease: a prospective cohort study. Inflamm Bowel Dis. 2017; 23:1643–1649.
Article18. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010; 341:c3369. doi:10.1136/bmj.c3369.
Article19. Scaioli E, Scagliarini M, Cardamone C, et al. Clinical application of faecal calprotectin in ulcerative colitis patients. Eur J Gastroenterol Hepatol. 2015; 27:1418–1424.
Article20. Taghvaei T, Maleki I, Nagshvar F, et al. Fecal calprotectin and ulcerative colitis endoscopic activity index as indicators of mucosal healing in ulcerative colitis. Intern Emerg Med. 2015; 10:321–328.
Article21. Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013; 7:e641–e651. doi: 10.1016/j.crohns.2013.05.005.
Article22. Lobatón T, Rodríguez-Moranta F, Lopez A, Sánchez E, Rodríguez-Alonso L, Guardiola J. A new rapid quantitative test for fecal calprotectin predicts endoscopic activity in ulcerative colitis. Inflamm Bowel Dis. 2013; 19:1034–1042.
Article23. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013; 19:332–341.
Article24. Bressler B, Panaccione R, Fedorak RN, Seidman EG. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol. 2015; 29:369–372.
Article25. Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002; 123:450–460.
Article26. Ikhtaire S, Shajib MS, Reinisch W, Khan WI. Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol. 2016; 51:434–446.27. Kawashima K, Ishihara S, Yuki T, et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016; 16:47. doi: 10.1186/s12876-016-0462-z.
Article28. Inokuchi T, Kato J, Hiraoka S, et al. Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn’s disease. Inflamm Bowel Dis. 2016; 22:1078–1085.
Article29. Yamaguchi S, Takeuchi Y, Arai K, et al. Fecal calprotectin is a clinically relevant biomarker of mucosal healing in patients with quiescent ulcerative colitis. J Gastroenterol Hepatol. 2016; 31:93–98.
Article30. Hanai H, Takeuchi K, Iida T, et al. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004; 49:1438–1443.
Article31. Yang SK, Hong M, Oh H, et al. Identification of loci at 1q21 and 16q23 that affect susceptibility to inflammatory bowel disease in Koreans. Gastroenterology. 2016; 151:1096–1099. e4.
Article32. Burri E, Manz M, Rothen C, Rossi L, Beglinger C, Lehmann FS. Monoclonal antibody testing for fecal calprotectin is superior to polyclonal testing of fecal calprotectin and lactoferrin to identify organic intestinal disease in patients with abdominal discomfort. Clin Chim Acta. 2013; 416:41–47.
Article33. Aomatsu T, Yoden A, Matsumoto K, et al. Fecal calprotectin is a useful marker for disease activity in pediatric patients with inflammatory bowel disease. Dig Dis Sci. 2011; 56:2372–2377.
Article34. Aomatsu T, Imaeda H, Matsumoto K, et al. Faecal chitinase 3-like-1: a novel biomarker of disease activity in paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2011; 34:941–948.
Article35. Li F, Ma J, Geng S, et al. Fecal calprotectin concentrations in healthy children aged 1-18 months. PLoS One. 2015; 10:e0119574. doi: 10.1371/journal.pone.0119574.
Article36. Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987; 92:1894–1898.
Article37. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19 Suppl A:5A–36A.
Article38. Amcoff K, Stridsberg M, Lampinen M, Magnuson A, Carlson M, Halfvarson J. Clinical implications of assay specific differences in f-calprotectin when monitoring inflammatory bowel disease activity over time. Scand J Gastroenterol. 2017; 52:344–350.
Article39. Lin WC, Wong JM, Tung CC, et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015; 21:13566–13573.
Article40. Fukunaga S, Kuwaki K, Mitsuyama K, et al. Detection of calprotectin in inflammatory bowel disease: fecal and serum levels and immunohistochemical localization. Int J Mol Med. 2018; 41:107–118.
Article41. Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016; 2:16014. doi: 10.1038/nrdp.2016.14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of fecal calprotectin in pediatric disease
- Fecal Calprotectin in Inflammatory Bowel Disease
- Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
- Home-based fecal calprotectin test is expected to play an important role in patients with inflammatory bowel diseases
- Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis