Anat Cell Biol.
2018 Dec;51(Suppl 1):S1-S12. 10.5115/acb.2018.51.S1.S1.
Histomorphometric study of rabbit's maxillary sinus augmentation with various graft materials
- Affiliations
-
- 1Department of Dentistry and Oral and Maxillofacial Surgery, Daegu Catholic University Medical Center, Daegu, Korea.
- 2Department of Anatomy, Catholic University of Daegu School of Medicine, Daegu, Korea. ysmoon@cu.ac.kr
- KMID: 2431626
- DOI: http://doi.org/10.5115/acb.2018.51.S1.S1
Abstract
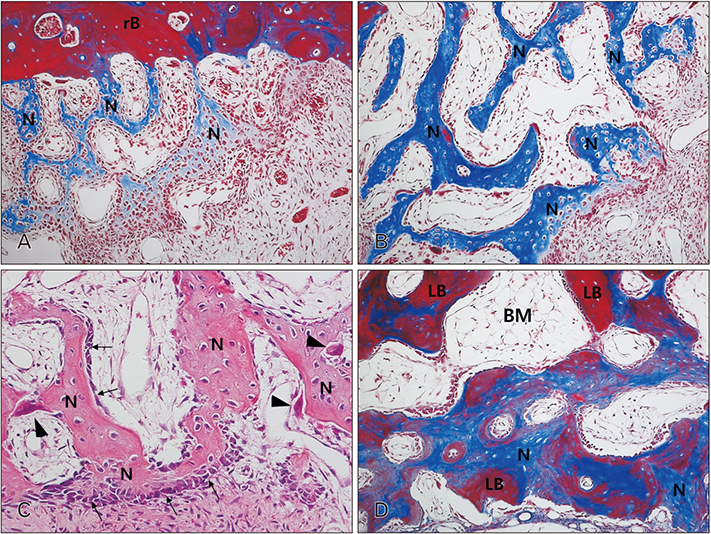
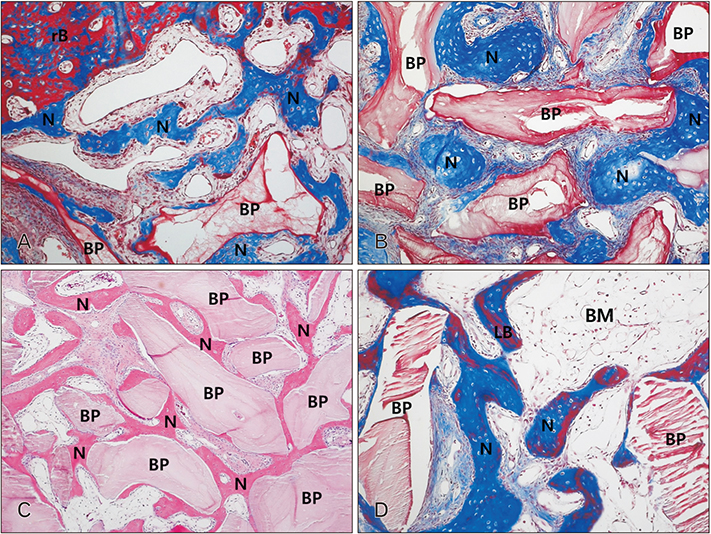
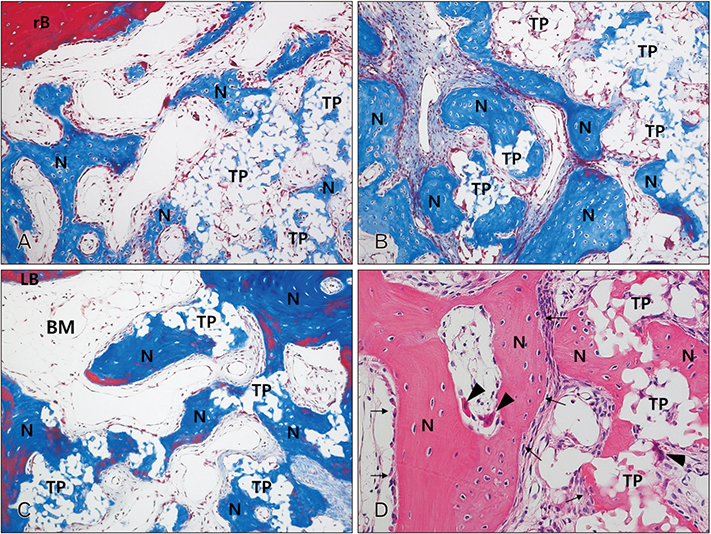
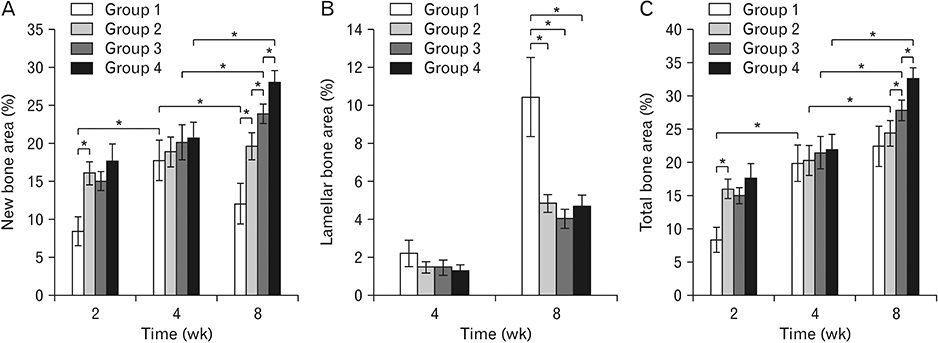
- The purpose of this animal study is to evaluate, by histomorphometric analysis, bone regeneration in rabbit's maxillary sinuses with blood clots alone, Bio-Oss, β-tricalcium phosphate (β-TCP), and demineralized tooth dentin (DTD) grafting. Bilateral sinus augmentation procedures were performed in 18 adult male rabbits. Rectangular replaceable bony windows were made with a piezoelectric thin saw insert. In the group 1, blood clots were filled; group 2, anorganic bovine graft (Bio-Oss) was grafted; group 3, β-TCP was grafted; group 4, DTD was grafted, and covered by replaceable bony windows. Animals were sacrificed at 2, 4, and 8 weeks after surgical procedure. The augmented sinuses were evaluated by histomorphometric analysis using hematoxylin and eosin and Masson's trichrome stains. Histologically, new bone formation was revealed along the elevated sinus membrane and all graft materials. The new bone area of the group 2 was significantly greater than the group 1, and of the group 3 was significantly greater than the group 2, and of the group 4 was significantly greater than the group 3 at 8 weeks with P < 0.05. The bone marrow area of group 1 was significantly greater than other groups at 8 weeks. The DTD area was significantly lesser than Bio-Oss or β-TCP particles area at 8 weeks. This present study suggests that DTD can be effective graft materials for bone regeneration of the maxillary sinus augmentation.
Keyword
MeSH Terms
Figure
Reference
-
1. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38:613–616.2. Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986; 30:207–229.3. Misch CE. Maxillary sinus augmentation for endosteal implants: organized alternative treatment plans. Int J Oral Implantol. 1987; 4:49–58.4. Chanavaz M. Maxillary sinus: anatomy, physiology, surgery, and bone grafting related to implantology: eleven years of surgical experience (1979–1990). J Oral Implantol. 1990; 16:199–209.5. Smiler DG, Johnson PW, Lozada JL, Misch C, Rosenlicht JL, Tatum OH Jr, Wagner JR. Sinus lift grafts and endosseous implants. Treatment of the atrophic posterior maxilla. Dent Clin North Am. 1992; 36:151–186.6. Hürzeler MB, Kirsch A, Ackermann KL, Quiñones CR. Reconstruction of the severely resorbed maxilla with dental implants in the augmented maxillary sinus: a 5-year clinical investigation. Int J Oral Maxillofac Implants. 1996; 11:466–475.7. Cordioli G, Mazzocco C, Schepers E, Brugnolo E, Majzoub Z. Maxillary sinus floor augmentation using bioactive glass granules and autogenous bone with simultaneous implant placement. Clinical and histological findings. Clin Oral Implants Res. 2001; 12:270–278.8. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007; 22:Suppl. 49–70.9. Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004; 25:987–994.
Article10. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989; 3:192–195.
Article11. De Menezes Oliveira MA, Torres CP, Gomes-Silva JM, Chinelatti MA, De Menezes FC, Palma-Dibb RG, Borsatto MC. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc Res Tech. 2010; 73:572–577.
Article12. Ozyuvaci H, Bilgic B, Firatli E. Radiologic and histomorphometric evaluation of maxillary sinus grafting with alloplastic graft materials. J Periodontol. 2003; 74:909–915.
Article13. Orsini G, Traini T, Scarano A, Degidi M, Perrotti V, Piccirilli M, Piattelli A. Maxillary sinus augmentation with Bio-Oss particles: a light, scanning, and transmission electron microscopy study in man. J Biomed Mater Res B Appl Biomater. 2005; 74:448–457.
Article14. Zijderveld SA, Zerbo IR, van den Bergh JP, Schulten EA, ten Bruggenkate CM. Maxillary sinus floor augmentation using a beta-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int J Oral Maxillofac Implants. 2005; 20:432–440.15. Chesmel KD, Branger J, Wertheim H, Scarborough N. Healing response to various forms of human demineralized bone matrix in athymic rat cranial defects. J Oral Maxillofac Surg. 1998; 56:857–863.
Article16. Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, Yamamoto G, Irie T, Tachikawa T, Maki K. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010; 81:1264–1272.
Article17. Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, Kim SY. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:496–503.
Article18. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967; 12:999–1008.
Article19. Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, Kusano K, Arisue M. Human acid-insoluble dentin with BMP-2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010; 118:438–441.
Article20. Kim YK, Lee J, Um IW, Kim KW, Murata M, Akazawa T, Mitsugi M. Tooth-derived bone graft material. J Korean Assoc Oral Maxillofac Surg. 2013; 39:103–111.
Article21. Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing: single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989; 84:640–646.
Article22. Jensen OT, Greer RO Jr, Johnson L, Kassebaum D. Vertical guided bone-graft augmentation in a new canine mandibular model. Int J Oral Maxillofac Implants. 1995; 10:335–344.
Article23. Smukler H, Barboza EP, Burliss C. A new approach to regeneration of surgically reduced alveolar ridges in dogs: a clinical and histologic study. Int J Oral Maxillofac Implants. 1995; 10:537–551.24. Leghissa GC, Zaffe D, Assenza B, Botticelli AR. Guided bone regeneration using titanium grids: report of 10 cases. Clin Oral Implants Res. 1999; 10:62–68.
Article25. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002; 13:405–409.
Article26. Small SA, Zinner ID, Panno FV, Shapiro HJ, Stein JI. Augmenting the maxillary sinus for implants: report of 27 patients. Int J Oral Maxillofac Implants. 1993; 8:523–528.27. Wiltfang J, Merten HA, Schlegel KA, Schultze-Mosgau S, Kloss FR, Rupprecht S, Kessler P. Degradation characteristics of alpha and beta tri-calcium-phosphate (TCP) in minipigs. J Biomed Mater Res. 2002; 63:115–121.28. Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997; 8:117–124.
Article29. Shin HI, Sohn DS. A method of sealing perforated sinus membrane and histologic finding of bone substitutes: a case report. Implant Dent. 2005; 14:328–333.
Article30. Walsh WR, Vizesi F, Michael D, Auld J, Langdown A, Oliver R, Yu Y, Irie H, Bruce W. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008; 29:266–271.31. Butler WT, Mikulski A, Urist MR, Bridges G, Uyeno S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977; 56:228–232.
Article32. Catanzaro-Guimarães SA, Catanzaro Guimarães BP, Garcia RB, Alle N. Osteogenic potential of autogenic demineralized dentin implanted in bony defects in dogs. Int J Oral Maxillofac Surg. 1986; 15:160–169.
Article33. Conover MA, Urist MR. Transmembrane bone morphogenesis by implants of dentin matrix. J Dent Res. 1979; 58:1911.
Article34. Gomes MF, dos Anjos MJ, Nogueira TO, Guimarães SA. Histologic evaluation of the osteoinductive property of autogenous demineralized dentin matrix on surgical bone defects in rabbit skulls using human amniotic membrane for guided bone regeneration. Int J Oral Maxillofac Implants. 2001; 16:563–571.35. Nordenram A, Bang G, Bernhoft CH. A clinical-radiographic study of allogenic demineralized dentin implants in cystic jaw cavities. Int J Oral Surg. 1975; 4:61–64.
Article36. Kim ES. Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Maxillofac Plast Reconstr Surg. 2015; 37:8.
Article37. Lee KH, Kim YK, Cho WJ, Um IW, Murata M, Mitsugi M. Autogenous tooth bone graft block for sinus augmentation with simultaneous implant installation: a technical note. J Korean Assoc Oral Maxillofac Surg. 2015; 41:284–289.
Article38. Kim YK, Lee JH, Um IW, Cho WJ. Guided bone regeneration using demineralized dentin matrix: long-term follow-up. J Oral Maxillofac Surg. 2016; 74:515.e1–515.e9.
Article39. Kim YK, Pang KM, Yun PY, Leem DH, Um IW. Long-term follow-up of autogenous tooth bone graft blocks with dental implants. Clin Case Rep. 2017; 5:108–118.
Article40. Lee J, Lee EY, Park EJ, Kim ES. An alternative treatment option for a bony defect from large odontoma using recycled demineralization at chairside. J Korean Assoc Oral Maxillofac Surg. 2015; 41:109–115.
Article41. Robinson C, Weatherell JA, Hallsworth AS. Variatoon in composition of dental enamel within thin ground tooth sections. Caries Res. 1971; 5:44–57.42. Bessho K, Tagawa T, Murata M. Comparison of bone matrix-derived bone morphogenetic proteins from various animals. J Oral Maxillofac Surg. 1992; 50:496–501.
Article43. Hoeppner LH, Secreto F, Jensen ED, Li X, Kahler RA, Westendorf JJ. Runx2 and bone morphogenic protein 2 regulate the expression of an alternative Lef1 transcript during osteoblast maturation. J Cell Physiol. 2009; 221:480–489.
Article44. Feng JQ, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni AB, D'Souza RN, Kozak CA, MacDougall M. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998; 273:9457–9464.
Article45. Ritchie HH, Ritchie DG, Wang LH. Six decades of dentinogenesis research: historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci. 1998; 106:Suppl 1. 211–220.46. Handschin AE, Egermann M, Trentz O, Wanner GA, Kock HJ, Zund G, Trentz OA. Cbfa-1 (Runx-2) and osteocalcin expression by human osteoblasts in heparin osteoporosis in vitro. Clin Appl Thromb Hemost. 2006; 12:465–472.47. Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004; 279:19141–19148.
Article48. Huggins CB, Urist MR. Dentin matrix transformation: rapid induction of alkaline phosphatase and cartilage. Science. 1970; 167:896–898.
Article49. Park M, Mah YJ, Kim DH, Kim ES, Park EJ. Demineralized deciduous tooth as a source of bone graft material: its biological and physicochemical characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015; 120:307–314.
Article50. Urist MR. Bone histogenesis and morphogenesis in implants of demineralized enamel and dentin. J Oral Surg. 1971; 29:88–102.51. Inoue T, Deporter DA, Melcher AH. Induction of cartilage and bone by dentin demineralized in citric acid. J Periodontal Res. 1986; 21:243–255.
Article52. Bang G. Induction of heterotopic bone formation by demineralized dentin in guinea pigs: antigenicity of the dentin matrix. J Oral Pathol. 1972; 1:172–185.
Article53. Bang G. Induction of heterotopic bone formation by demineralized dentin: an experimental model in guinea pigs. Scand J Dent Res. 1973; 81:240–250.
Article54. Urist MR, Dowell TA, Hay PH, Strates BS. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968; 59:59–96.
Article55. Reddi AH, Anderson WA. Collagenous bone matrix-induced endochondral ossification hemopoiesis. J Cell Biol. 1976; 69:557–572.
Article56. Ike M, Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998; 24:124–132.
Article57. Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006; 27:2798–2805.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maxillary sinus bone graft using particulated ramal autobone and bovine bone
- Vertical Augmentation of Maxillary Posterior Alveolar Ridge Using Allogenic Block Bone Graft and Simultaneous Maxillary Sinus Graft
- A Double Layers Technique for Maxillary Sinus Augmentation with Demineralized and Mineralized Bone Graft Materials
- Delayed Occurrence of Maxillary Sinusitis after Simultaneous Maxillary Sinus Augmentation and Implant: A Case Report and Literature Review
- SINUS GRAFT AND VERTICAL AUGMENTATION OF MAXILLARY POSTERIOR ALVEOLAR RIDGE USING MANDIBULAR RAMAL BLOCK BONE GRAFT