Korean J Phys Anthropol.
2018 Dec;31(4):143-149. 10.11637/kjpa.2018.31.4.143.
Inhibitory Effects of CD99-derived Peptide CD99CRIII3 on the Extravasation of Monocytes and Inflammatory Reactions in Contact Dermatitis Mouse Model
- Affiliations
-
- 1Department of Anatomy and Cell Biology, School of Medicine, Kangwon National University, Korea. insitu@kangwon.ac.kr
- KMID: 2430459
- DOI: http://doi.org/10.11637/kjpa.2018.31.4.143
Abstract
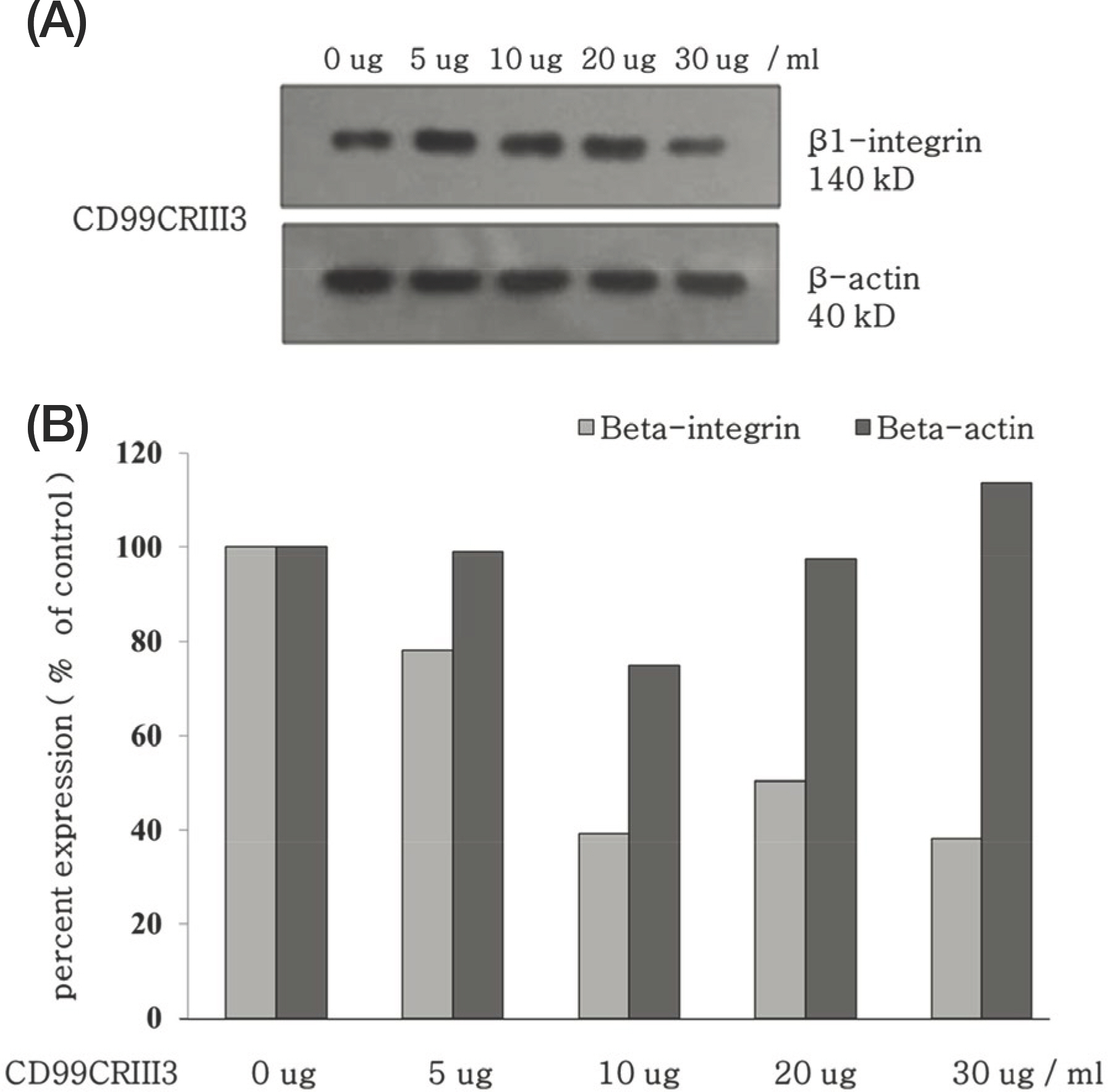
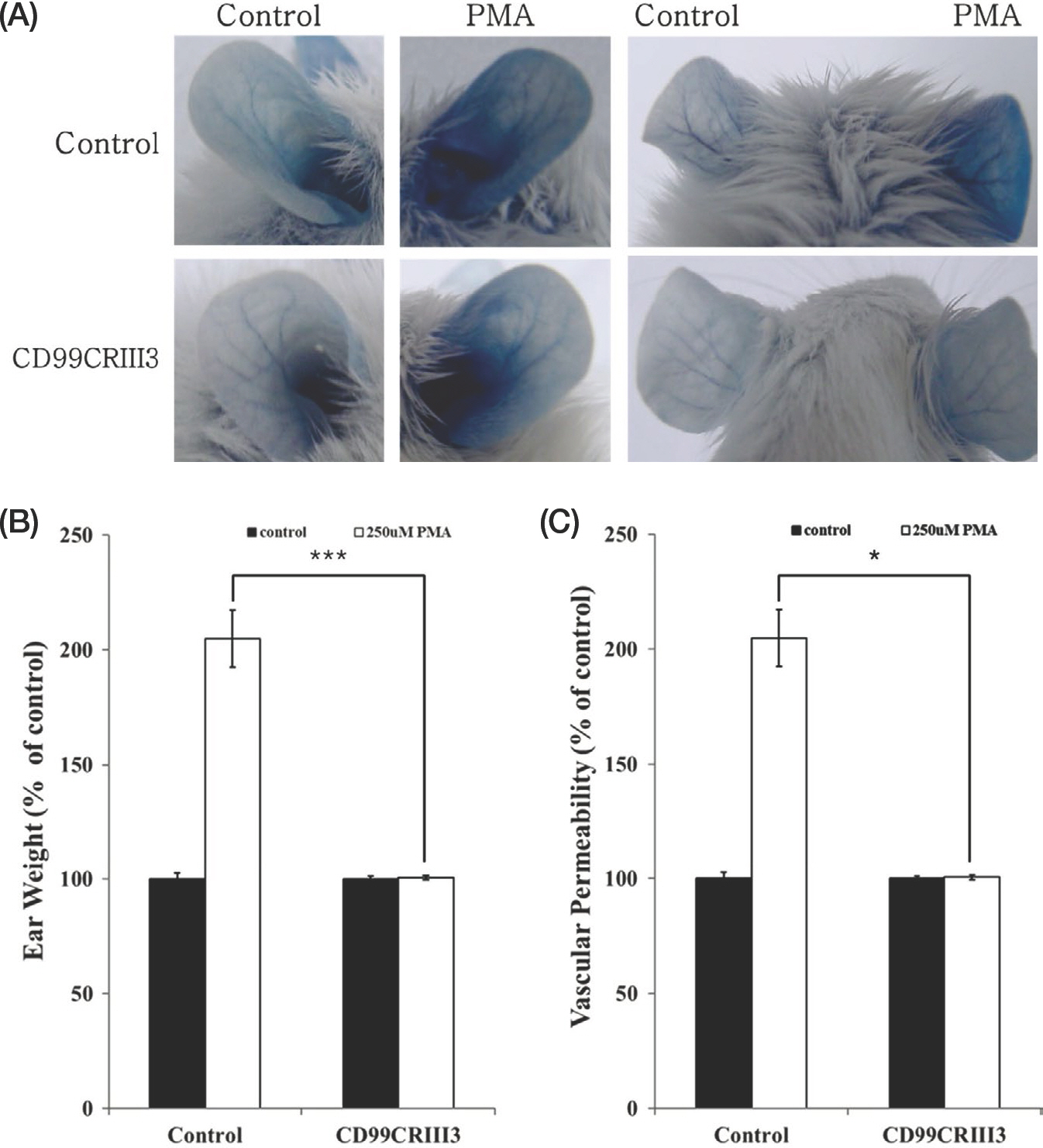
- Leucocyte extravasation has been known to play an important role in inflammatory reactions including contact dermatitis. Previous studies suggested that CD99 regulates β1 integrin activity and may be a novel therapeutic target molecule for inflammatory diseases. In this study, the effects of CD99-derived peptide, CD99CRIII3, on inflammatory reactions in contact dermatitis mouse model were investigated. CD99CRIII3 decreased β1-integrin activity in human monocytic U937 cells. CD99CRIII3 inhibited the adhesion of U937 monocytes to human umbilical vein endothelial cells and their extravasation through human umbilical vein endothelial cells. CD99CRIII3 reduced inflammation in the phorbol myristate acetate-induced contact dermatitis mice in a dose-dependent manner. These results indicate that CD99CRIII3 suppresses the extravasation of monocytes and inflammatory reactions in the animal model of the contact dermatitis, suggesting that CD99CRIII3 could be a new drug candidate against inflammatory skin diseases.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003; 361:151–60.
Article2. Giustizieri ML, Mascia F, Frezzolini A, De Pità O, Chinni LM, Giannetti A, et al. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol. 2001; 107:871–7.
Article3. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004; 113:651–7.
Article4. Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003; 112:1195–202.5. Beresford L, Orange O, Bell EB, Miyan JA. Nerve fibres are required to evoke a contact sensitivity response in mice. Immunology. 2004; 111:118–25.
Article6. Levy R, Dilley J, Fox RI, Warnke R. A human thymus-leu-kemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A. 1979; 76:6552–56.
Article7. Waclavicek M, Majdic O, Stulnig T, Berger M, SunderPlassmann R, Zlabinger GJ, et al. CD99 engagement on human peripheral blood T cells results in TCR/CD3-dependent cellular activation and allows for Th1-restricted cytokine production. J Immunol. 1998; 161:4671–78.8. Sohn HW, Shin YK, Lee IS, Bae YM, Suh YH, Kim M, et al. CD99 regulates the transport of MHC class I molecules from the Golgi complex to the cell surface. J Immunol. 2001; 166:787–94.
Article9. Sciandra M, Marino MT, Manara MC, Guerzoni C, Grano M, Oranger A, et al. CD99 drives terminal differentiation of osteosarcoma cells by acting as a spatial regulator of ERK 1/2. J. Bone Miner. Res. 2014; 29:1295–309.10. Sohn HW, Choi EY, Kim SH, Lee IS, Chung DH, Sung UA, et al. Engagement of CD99 induces apoptosis through a calcineurin-independent pathway in Ewing's sarcoma cells. Am J Pathol. 1998; 153:1937–45.
Article11. Husak Z, Printz D, Schumich A, Potschger U, Dworzak MN. Death induction by CD99 ligation in TEL/AML1-positive acute lymphoblastic leukemia and normal B cell precursors. J Leukoc Biol. 2010; 88:405–12.
Article12. Husak Z, Dworzak MN. CD99 ligation upregulates HSP70 on acute lymphoblastic leukemia cells and concomitantly increases NK cytotoxicity. Cell Death Dis. 2012; 3:e425.
Article13. Bernard A, Aubrit F, Raynal B, Pham D, Boumsell L. A T cell surface molecule different from CD2 is involved in spontaneous rosette formation with erythrocytes. J Immunol. 1988; 140:1802–07.14. Gelin C, Aubrit F, Phalipon A, Raynal B, Cole S, Kaczorek M, et al. The E2 antigen, a 32 kd glycoprotein involved in T-cell adhesion processes, is the MIC2 gene product. EMBO J. 1989; 8:3253–59.
Article15. Bernard G, Zoccola D, Deckert M, Breittmayer JP, Aussel C, Bernard A. The E2 molecule (CD99) specifically triggers homotypic aggregation of CD4+ CD8+ thymocytes. J Immunol. 1995; 154:26–32.16. Hahn JH, Kim MK, Choi EY, Kim SH, Sohn HW, Ham DI, et al. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. 1997; 159:2250–58.17. Hahn MJ, Yoon SS, Sohn HW, Song HG, Park SH, Kim TJ. Differential activation of MAP kinase family members triggered by CD99 engagement. FEBS Lett. 2000; 470:350–4.
Article18. Lee KJ, Kim Y, Yoo YH, Kim MS, Lee SH, Kim CG, et al. CD99-Derived Agonist Ligands Inhibit Fibronectin-In-duced Activation of β1 Integrin through the Protein Kinase A/SHP2/Extracellular Signal-Regulated Kinase/PTPN12/Focal Adhesion Kinase Signaling Pathway. Mol Cell Biol. 2017; 37:675–16.
Article19. Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat J Immunol. 2002; 3:143–50.
Article20. Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007; 178:1136–43.
Article21. Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood. 2004; 104:3205–13.
Article22. Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, et al. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006; 281:34833–47.
Article23. Cotter TG, Keeling PJ, Henson PM. A monoclonal antibody-inhibiting FMLP-induced chemotaxis of human neutrophils. J Immunol. 1981; 127:2241–5.24. Wu BC, Lee AH, Hancock REW. Mechanisms of the Innate Defense Regulator Peptide-1002 Anti-Inflammatory Activity in a Sterile Inflammation Mouse Model. J Immunol. 2017; 199:3592–3603.
Article25. Sahu RP, Rezania S, Ocana JA, DaSilva-Arnold SC, Brad-ish JR, Richey JD, et al. Topical application of a platelet activating factor receptor agonist suppresses phorbol es-ter-induced acute and chronic inflammation and has cancer chemopreventive activity in mouse skin. PLoS One. 2014; 9:e111608.
Article26. Kim MJ, Kim YY, Choi YA, Baek MC, Lee B, Park PH, et al. Elaeocarpusin Inhibits Mast Cell-Mediated Allergic Inflammation.Front Pharmacol. 2018; 9:591.27. Lee KJ, Lim D, Yoo YH, Park EJ, Lee SH, Yadav BK, et al. Paired Ig-like type 2 receptor-derived agonist ligands ameliorate inflammatory reactions by downregulating β1 integrin activity. Mol Cells. 2016; 39:557–65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Irritant Contact Dermafitis Due to Ranuculus japonicus
- Allergic contact dermatitis to hydroquinone in bleaching cream
- A Patient with Delayed Contact Dermatitis to Coral and She Displayed Superficial Granuloma
- Systemic Contact Dermatitis from Propolis Ingestion
- A Case of Allergic Contact Dermatitis from Calcipotriol(Daivonex(R))