Allergy Asthma Immunol Res.
2018 Jan;10(1):62-76. 10.4168/aair.2018.10.1.62.
Detecting Allergens From Black Tiger Shrimp Penaeus monodon That Can Bind and Cross-link IgE by ELISA, Western Blot, and a Humanized Rat Basophilic Leukemia Reporter Cell Line RS-ATL8
- Affiliations
-
- 1Graduate Program in Biotechnology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand.
- 2Department of Biology, Faculty of Science, Mahidol University, Bangkok, Thailand.
- 3Allergy&Immunology Unit, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
- 4Institute of Biotechnology and Genetic Engineering, Chulalongkorn University, Bangkok, Thailand.
- 5National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency, Pathum Thani, Thailand.
- 6Department of Microbiology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand. tanapat.p@chula.ac.th
- 7Chula-Vaccine Research Center, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
- 8Center of Excellence in Immunology and Immune-mediated Diseases, Chulalongkorn University, Bangkok, Thailand.
- KMID: 2428851
- DOI: http://doi.org/10.4168/aair.2018.10.1.62
Abstract
- BACKGROUND
Black tiger shrimp Penaeus monodon is one of the common causes of shellfish allergy that is increasing worldwide. One of the important problems in the management of shellfish allergy is the lack of accurate diagnostic assay because the biological and immunological properties of allergens in black tiger shrimp have not been well characterized. This study aims to detect proteins with the ability to bind and cross-link immunoglobulin E (IgE) from black tiger shrimp by enzyme-linked immunosorbent assay (ELISA), Western blot, and a humanized rat basophilic leukemia reporter cell line RS-ATL8.
METHODS
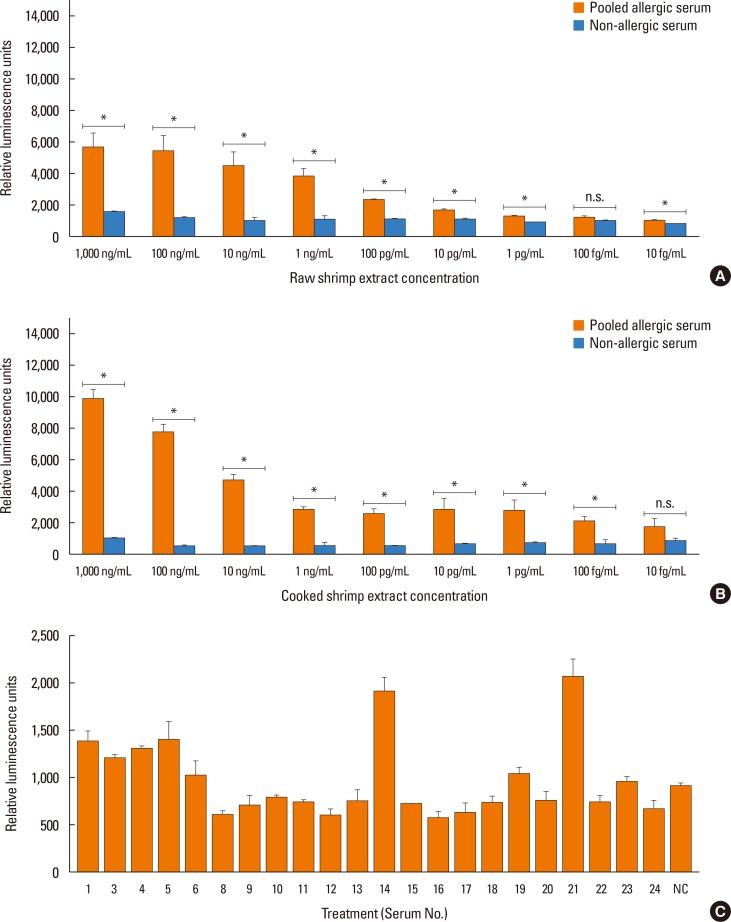
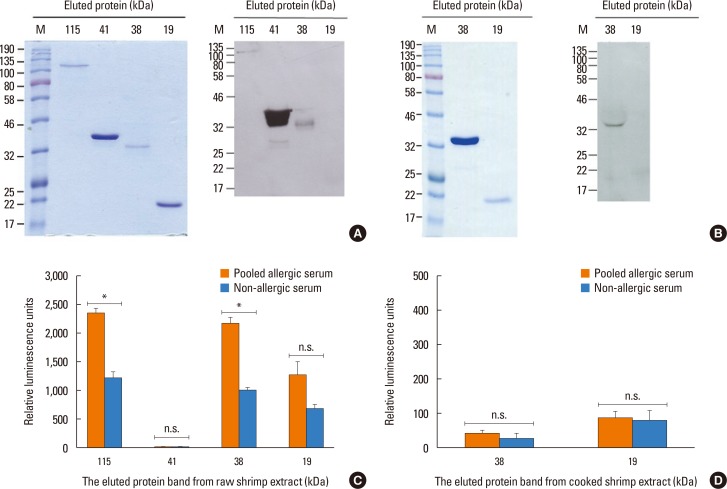
Sera from shrimp allergic subjects were subjected to ELISA and Western blots using raw or cooked shrimp extract as antigens. Pooled sera were used to sensitize the RS-ATL8 reporter cell line and cells were activated by shrimp extract. Eluted protein extracts separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) were tested on the RS-ATL8 cell line and subjected to mass spectrometry to identify potential candidate allergens.
RESULTS
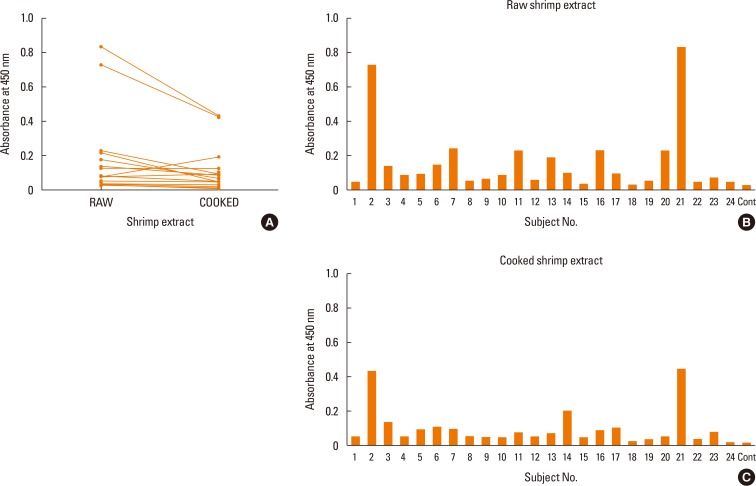
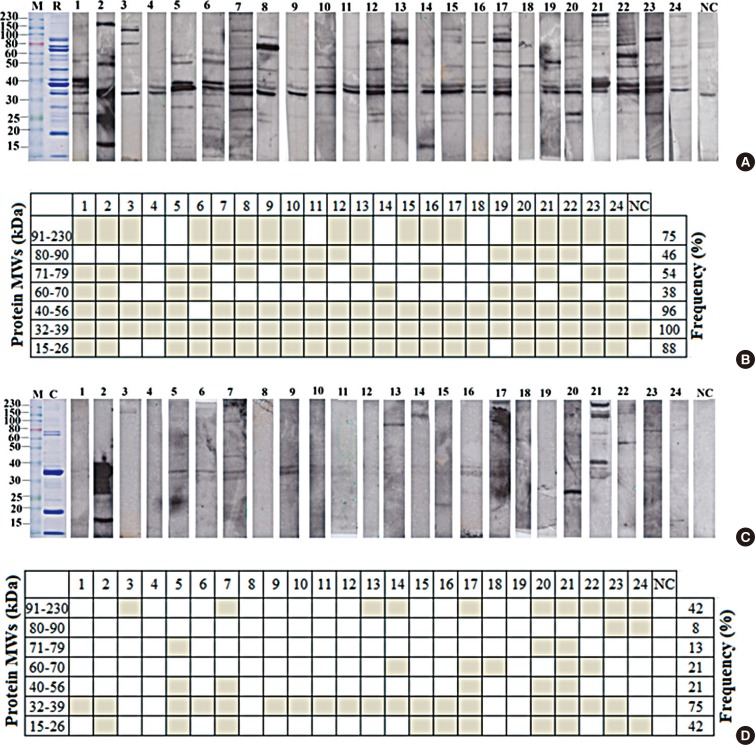
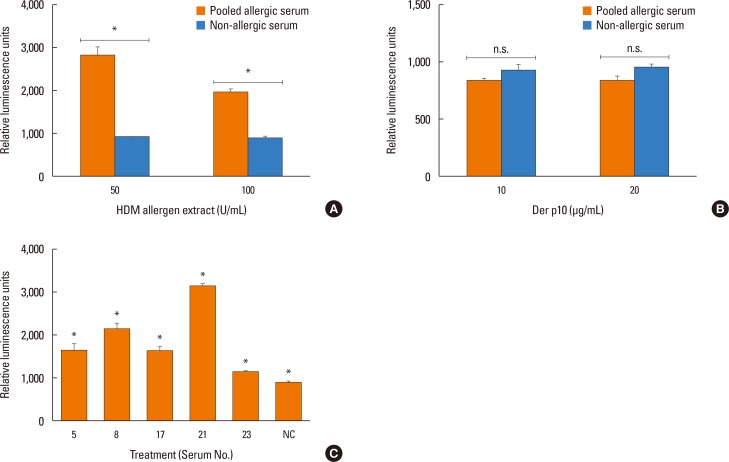
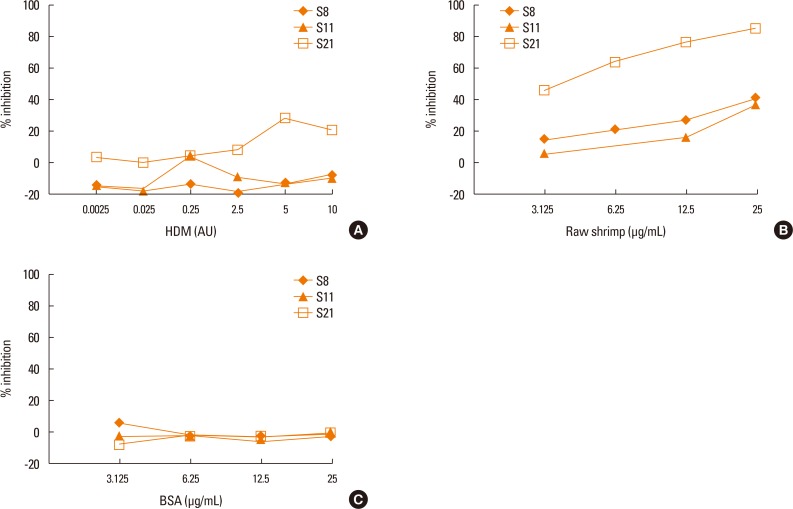
Allergic sera reacted stronger to raw shrimp extract than cooked shrimp extract (P=0.009). Western blot demonstrated that major IgE reactivity protein bands were at 32-39 kDa and 91-230 kDa in both raw and cooked shrimp extracts. The eluted protein bands at the molecular weight of 38 and 115 kDa from raw shrimp extract induced IgE cross-linking as assayed by the RS-ATL8 cell line. These protein bands were subjected to mass spectrometry for analysis. Ubiquitin-activating enzyme and crustacyanin were identified as potential candidate novel shrimp allergens.
CONCLUSIONS
The RS-ATL8 reporter cell line can be used to identify potential new shrimp allergens that can functionally cross-link IgE and induce mast cell degranulation.
MeSH Terms
-
Allergens*
Animals
Basophils*
Blotting, Western*
Cell Line*
Electrophoresis, Polyacrylamide Gel
Enzyme-Linked Immunosorbent Assay*
Humans*
Immunoglobulin E*
Immunoglobulins
Leukemia*
Mass Spectrometry
Mast Cells
Molecular Weight
Penaeidae*
Rats*
Shellfish Hypersensitivity
Sodium Dodecyl Sulfate
Tigers*
Ubiquitin-Activating Enzymes
Allergens
Immunoglobulin E
Immunoglobulins
Sodium Dodecyl Sulfate
Ubiquitin-Activating Enzymes
Figure
Reference
-
2. Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013; 3:3–14. PMID: 23403837.
Article3. Daul CB, Morgan JE, Hughes J, Lehrer SB. Provocation-challenge studies in shrimp-sensitive individuals. J Allergy Clin Immunol. 1988; 81:1180–1186. PMID: 3379230.
Article4. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008; 100:S1–S148.
Article5. Musmand JJ, Daul CB, Lehrer SB. Crustacea allergy. Clin Exp Allergy. 1993; 23:722–732. PMID: 10779302.
Article6. Nakamura R, Uchida Y, Higuchi M, Nakamura R, Tsuge I, Urisu A, et al. A convenient and sensitive allergy test: IgE crosslinking-induced luciferase expression in cultured mast cells. Allergy. 2010; 65:1266–1273. PMID: 20374229.
Article7. Faber MA, Pascal M, El Kharbouchi O, Sabato V, Hagendorens MM, Decuyper II, et al. Shellfish allergens: tropomyosin and beyond. Allergy. 2017; 72:842–848. PMID: 28027402.
Article8. Leung PS, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, et al. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol. 1994; 94:882–890. PMID: 7963157.9. Wong L, Huang CH, Lee BW. Shellfish and house dust mite allergies: is the link tropomyosin? Allergy Asthma Immunol Res. 2016; 8:101–106. PMID: 26739402.
Article10. Vogel L, Lüttkopf D, Hatahet L, Haustein D, Vieths S. Development of a functional in vitro assay as a novel tool for the standardization of allergen extracts in the human system. Allergy. 2005; 60:1021–1028. PMID: 15969682.11. Pedrosa M, Boyano-Martínez T, García-Ara C, Quirce S. Shellfish allergy: a comprehensive review. Clin Rev Allergy Immunol. 2015; 49:203–216. PMID: 24870065.
Article12. Nakamura R, Ishiwatari A, Higuchi M, Uchida Y, Nakamura R, Kawakami H, et al. Evaluation of the luciferase assay-based in vitro elicitation test for serum IgE. Allergol Int. 2012; 61:431–437. PMID: 22722812.13. Falcone FH, Alcocer MJ, Okamoto-Uchida Y, Nakamura R. Use of humanized rat basophilic leukemia reporter cell lines as a diagnostic tool for detection of allergen-specific IgE in allergic patients: time for a reappraisal? Curr Allergy Asthma Rep. 2015; 15:67. PMID: 26452547.
Article14. Abramovitch JB, Kamath S, Varese N, Zubrinich C, Lopata AL, O'Hehir RE, et al. IgE reactivity of blue swimmer crab (Portunus pelagicus) tropomyosin, Por p 1, and other allergens; cross-reactivity with black tiger prawn and effects of heating. PLoS One. 2013; 8:e67487. PMID: 23840718.15. Abugoch L, Tapia C, Plasencia D, Pastor A, Castro-Mandujano O, López L, et al. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J Sci Food Agric. 2016; 96:619–626. PMID: 25678380.
Article16. Jeong KY, Hwang H, Lee J, Lee IY, Kim DS, Hong CS, et al. Allergenic characterization of tropomyosin from the dusky brown cockroach, Periplaneta fuliginosa. Clin Diagn Lab Immunol. 2004; 11:680–685. PMID: 15242941.17. Paemanee A, Wikan N, Roytrakul S, Smith DR. Application of gelC-MS/MS to proteomic profiling of chikungunya virus infection: preparation of peptides for analysis. Methods Mol Biol. 2016; 1426:179–193. PMID: 27233271.
Article18. Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999; 20:3551–3567. PMID: 10612281.
Article19. Vaudel M, Burkhart JM, Zahedi RP, Oveland E, Berven FS, Sickmann A, et al. PeptideShaker enables reanalysis of MS-derived proteomics data sets. Nat Biotechnol. 2015; 33:22–24. PMID: 25574629.
Article20. Vaudel M, Barsnes H, Berven FS, Sickmann A, Martens L. Search-GUI: an open-source graphical user interface for simultaneous OMSSA and X!Tandem searches. Proteomics. 2011; 11:996–999. PMID: 21337703.
Article21. Sahabudin S, Misnan R, Yadzir ZH, Mohamad J, Abdullah N, Bakhtiar F, et al. Identification of major and minor allergens of black tiger prawn (Penaeus monodon) and king prawn (Penaeus latisulcatus). Malays J Med Sci. 2011; 18:27–32.22. Abdel Rahman AM, Kamath S, Lopata AL, Helleur RJ. Analysis of the allergenic proteins in black tiger prawn (Penaeus monodon) and characterization of the major allergen tropomyosin using mass spectrometry. Rapid Commun Mass Spectrom. 2010; 24:2462–2470. PMID: 20658686.23. Samson KT, Chen FH, Miura K, Odajima Y, Iikura Y, Naval Rivas M, et al. IgE binding to raw and boiled shrimp proteins in atopic and nonatopic patients with adverse reactions to shrimp. Int Arch Allergy Immunol. 2004; 133:225–232. PMID: 14976390.
Article24. Paschke A, Besler M. Stability of bovine allergens during food processing. Ann Allergy Asthma Immunol. 2002; 89:16–20. PMID: 12487199.
Article25. Nakamura A, Sasaki F, Watanabe K, Ojima T, Ahn DH, Saeki H. Changes in allergenicity and digestibility of squid tropomyosin during the Maillard reaction with ribose. J Agric Food Chem. 2006; 54:9529–9534. PMID: 17147442.
Article26. Liu GM, Cheng H, Nesbit JB, Su WJ, Cao MJ, Maleki SJ. Effects of boiling on the IgE-binding properties of tropomyosin of shrimp (Litopenaeus vannamei). J Food Sci. 2010; 75:T1–T5. PMID: 20492208.27. Carnés J, Ferrer A, Huertas AJ, Andreu C, Larramendi CH, Fernández-Caldas E. The use of raw or boiled crustacean extracts for the diagnosis of seafood allergic individuals. Ann Allergy Asthma Immunol. 2007; 98:349–354. PMID: 17458431.
Article28. Gámez C, Zafra MP, Sanz V, Mazzeo C, Ibáñez MD, Sastre J, et al. Simulated gastrointestinal digestion reduces the allergic reactivity of shrimp extract proteins and tropomyosin. Food Chem. 2015; 173:475–481. PMID: 25466048.
Article29. Usui M, Harada A, Ishimaru T, Sakumichi E, Saratani F, Sato-Minami C, et al. Contribution of structural reversibility to the heat stability of the tropomyosin shrimp allergen. Biosci Biotechnol Biochem. 2013; 77:948–953. PMID: 23649255.
Article30. Daul CB, Slattery M, Reese G, Lehrer SB. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. 1994; 105:49–55. PMID: 7916224.31. Khanaruksombat S, Srisomsap C, Chokchaichamnankit D, Punyarit P, Phiriyangkul P. Identification of a novel allergen from muscle and various organs in banana shrimp (Fenneropenaeus merguiensis). Ann Allergy Asthma Immunol. 2014; 113:301–306. PMID: 24996992.32. Gámez C, Zafra M, Boquete M, Sanz V, Mazzeo C, Ibáñez MD, et al. New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res. 2014; 58:1915–1925. PMID: 24978201.
Article33. Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003; 170:445–453. PMID: 12496430.
Article34. Bodinier M, Brossard C, Triballeau S, Morisset M, Guérin-Marchand C, Pineau F, et al. Evaluation of an in vitro mast cell degranulation test in the context of food allergy to wheat. Int Arch Allergy Immunol. 2008; 146:307–320. PMID: 18367844.35. Eberlein-König B, Varga R, Mempel M, Darsow U, Behrendt H, Ring J. Comparison of basophil activation tests using CD63 or CD203c expression in patients with insect venom allergy. Allergy. 2006; 61:1084–1085. PMID: 16918511.
Article36. Bridts CH, Sabato V, Mertens C, Hagendorens MM, De Clerck LS, Ebo DG. Flow cytometric allergy diagnosis: basophil activation techniques. Methods Mol Biol. 2014; 1192:147–159. PMID: 25149490.
Article37. Wan D, Wang X, Nakamura R, Alcocer MJ, Falcone FH. Use of humanized rat basophil leukemia (RBL) reporter systems for detection of allergen-specific IgE sensitization in human serum. Methods Mol Biol. 2014; 1192:177–184. PMID: 25149492.
Article38. Dibbern DA Jr, Palmer GW, Williams PB, Bock SA, Dreskin SC. RBL cells expressing human Fc epsilon RI are a sensitive tool for exploring functional IgE-allergen interactions: studies with sera from peanut-sensitive patients. J Immunol Methods. 2003; 274:37–45. PMID: 12609531.39. Wan D, Ludolf F, Alanine DG, Stretton O, Ali Ali E, Al-Barwary N, et al. Use of humanised rat basophilic leukaemia cell line RS-ATL8 for the assessment of allergenicity of Schistosoma mansoni proteins. PLoS Negl Trop Dis. 2014; 8:e3124. PMID: 25254513.40. Sotkovský P, Hubálek M, Hernychová L, Novák P, Havranová M, Setinová I, et al. Proteomic analysis of wheat proteins recognized by IgE antibodies of allergic patients. Proteomics. 2008; 8:1677–1691. PMID: 18340628.
Article41. Kamath SD, Rahman AM, Voskamp A, Komoda T, Rolland JM, O'Hehir RE, et al. Effect of heat processing on antibody reactivity to allergen variants and fragments of black tiger prawn: a comprehensive allergenomic approach. Mol Nutr Food Res. 2014; 58:1144–1155. PMID: 24420734.
Article42. Nagai Y, Kaneda S, Nomura K, Yasuda H, Seno T, Yamao F. Ubiquitin-activating enzyme, E1, is phosphorylated in mammalian cells by the protein kinase Cdc2. J Cell Sci. 1995; 108:2145–2152. PMID: 7673335.
Article43. Ferrari M, Folli C, Pincolini E, McClintock TS, Rössle M, Berni R, et al. Structural characterization of recombinant crustacyanin subunits from the lobster Homarus americanus. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012; 68:846–853.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification and characterization of shrimp allergens in Korea
- Viral Disease of the Cultured Penaeus chinensis and Penaeus japonicus
- Changes of allergenecity of salted and fermented shrimp
- Specific IgE determination to shrimp (Metapenaeus joyneri) and identification of its allergen
- Cross - reactivity between pollens in patients sensitlzed to multiple pollens