A Retrospective Study of Clinical Response Predictors in Subcutaneous Allergen Immunotherapy With House Dust Mites for Allergic Rhinitis
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. ye9007@ajou.ac.kr
- 2Clinical Trial Center, Ajou University Medical Center, Suwon, Korea.
- KMID: 2428846
- DOI: http://doi.org/10.4168/aair.2018.10.1.18
Abstract
- PURPOSE
House dust mites (HDM) are major allergens that cause allergic rhinitis (AR). Allergen-specific subcutaneous immunotherapy (SCIT) has been shown to be clinically beneficial in many clinical trials. Such trials, however, are not reflective of all patient populations. The aim of this study was to describe the efficacy and safety of SCIT in routine clinical practice in Korean adults with AR sensitized to HDM.
METHODS
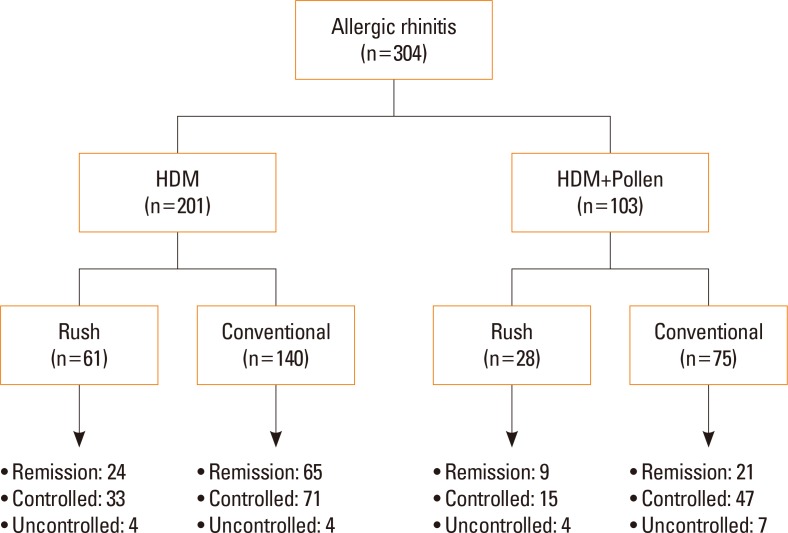
We reviewed medical records of 304 patients with AR treated at an allergy clinic of a tertiary hospital using SCIT with aluminum hydroxide-adsorbed allergen extract targeting HDM alone or with pollens for at least 1 year from 2000 to 2012. Patients with asthma were excluded. Rates of remission, defined as no further requirement of maintenance medication, over time were determined by means of life tables and extension of survival analysis. Specific immunoglobulin E (IgE) levels to HDM were categorized into 6 classes.
RESULTS
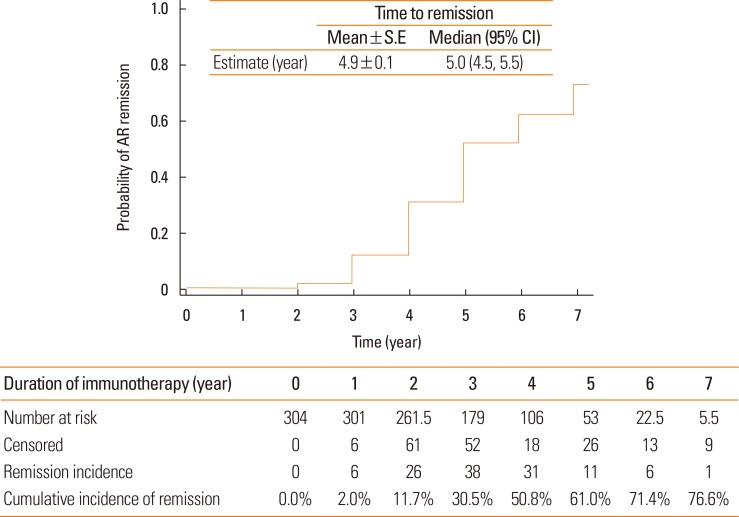
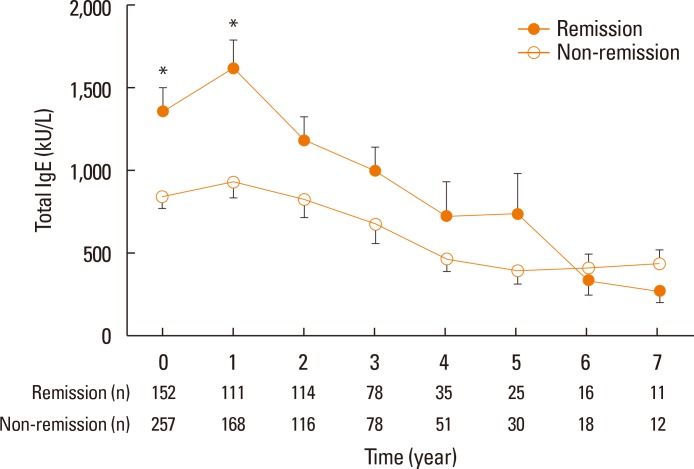
The mean time until achieving remission was 4.9±0.1 years, and the cumulative incidence of remission from AR was 76.6%. Severe AR (odds ratio [OR], 0.40; 95% confidence interval [CI], 0.23-0.69; P=0.001), specific IgE levels to HDM ≥17.5 kU/L (OR, 1.85; 95% CI, 1.01-3.37; P=0.045), and duration of immunotherapy ≥3 years (OR, 7.37; 95% CI, 3.50-15.51; P<0.001) were identified as significant predictors of clinical remission during SCIT for patients with AR sensitized to HDM. Overall, 73 patients (24.0%) experienced adverse reactions to SCIT, and only 1 case of anaphylaxis (0.3%) developed.
CONCLUSIONS
SCIT with HDM was found to be effective and safe for patients with AR. Specific IgE levels to HDM and a duration of SCIT ≥3 years may be predictors of clinical responses to SCIT in AR patients.
MeSH Terms
-
Adult
Allergens
Aluminum
Anaphylaxis
Asthma
Desensitization, Immunologic*
Dust*
Humans
Hypersensitivity
Immunoglobulin E
Immunoglobulins
Immunotherapy
Incidence
Life Tables
Medical Records
Pollen
Pyroglyphidae*
Retrospective Studies*
Rhinitis, Allergic*
Tertiary Care Centers
Allergens
Aluminum
Dust
Immunoglobulin E
Immunoglobulins
Figure
Cited by 7 articles
-
Efficacy and Safety of Subcutaneous Allergen Immunotherapy for Allergic Rhinitis
Myung Hyun Sohn
Allergy Asthma Immunol Res. 2018;10(1):1-3. doi: 10.4168/aair.2018.10.1.1.Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy
Wat Mitthamsiri, Panitan Pradubpongsa, Atik Sangasapaviliya, Tadech Boonpiyathad
Allergy Asthma Immunol Res. 2018;10(6):662-674. doi: 10.4168/aair.2018.10.6.662.Comparison of Long-term Efficacy of Subcutaneous Immunotherapy in Pediatric and Adult Patients With Allergic Rhinitis
Yanran Huang, Chengshuo Wang, Feifei Cao, Yan Zhao, Hongfei Lou, Luo Zhang
Allergy Asthma Immunol Res. 2019;11(1):68-78. doi: 10.4168/aair.2019.11.1.68.Safety of Ultra-rush Schedule of Subcutaneous Allergen Immunotherapy With House Dust Mite Extract Conducted in an Outpatient Clinic in Patients With Atopic Dermatitis and Allergic Rhinitis
So-Hee Lee, Myoung-Eun Kim, Yoo Seob Shin, Young-Min Ye, Hae-Sim Park, Dong-Ho Nahm
Allergy Asthma Immunol Res. 2019;11(6):846-855. doi: 10.4168/aair.2019.11.6.846.Efficacy of Allergen Immunotherapy for Allergic Asthma in Real World Practice
Hyo-In Rhyou, Young-Hee Nam
Allergy Asthma Immunol Res. 2020;12(1):99-109. doi: 10.4168/aair.2020.12.1.99.Factors Associated with Adherence to Allergen Specific Subcutaneous Immunotherapy
Ji-Ho Lee, So-Hee Lee, Ga-Young Ban, Young-Min Ye, Dong-Ho Nahm, Hae-Sim Park, Yoo Seob Shin
Yonsei Med J. 2019;60(6):570-577. doi: 10.3349/ymj.2019.60.6.570.Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation
Luciana S. Aragão-França, Viviane C. J. Rocha, Andre Cronemberger-Andrade, F. H. B. Costa, José Fernandes Vasconcelos, Daniel Abensur Athanazio, Daniela Nascimento Silva, E. S. Santos, Cássio Santana Meira, C. F. Araújo, Jéssica Vieira Cerqueira, Fabíola Cardillo, Neuza Maria Alcântara-Neves, Milena Botelho Pereira Soares, Lain C. Pontes-de-Carvalho
Allergy Asthma Immunol Res. 2018;10(4):406-419. doi: 10.4168/aair.2018.10.4.406.
Reference
-
1. Pawankar R, Bunnag C, Khaltaev N, Bousquet J. Allergic rhinitis and its impact on asthma in Asia Pacific and the ARIA update 2008. World Allergy Organ J. 2012; 5:S212–S217. PMID: 23268481.
Article2. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004; 24:758–764. PMID: 15516669.
Article3. Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015; 372:456–463. PMID: 25629743.4. Lee KS, Yum HY, Sheen YH, Park YM, Lee YJ, Choi BS, et al. Comorbidities and phenotypes of rhinitis in Korean children and adolescents: a cross-sectional, multicenter study. Allergy Asthma Immunol Res. 2017; 9:70–78. PMID: 27826964.
Article5. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160. PMID: 18331513.6. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127:S1–S55. PMID: 21122901.7. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID: 26922928.
Article8. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014; 133:621–631. PMID: 24581429.
Article9. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016; 137:339–349. PMID: 26853126.
Article10. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015; 136:556–568. PMID: 26162571.
Article11. Schmitt J, Schwarz K, Stadler E, Wüstenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015; 136:1511–1516. PMID: 26371838.
Article12. Bodtger U, Linneberg A. Remission of allergic rhinitis: an 8-year observational study. J Allergy Clin Immunol. 2004; 114:1384–1388. PMID: 15577841.
Article13. Rottem M, Egbarya A. Subcutaneous immunotherapy in Northern Israel: efficacy and safety. Isr Med Assoc J. 2014; 16:539–543. PMID: 25351009.14. Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. 2006; 61:191–197. PMID: 16409195.
Article15. Asero R. Efficacy of injection immunotherapy with ragweed and birch pollen in elderly patients. Int Arch Allergy Immunol. 2004; 135:332–335. PMID: 15564775.
Article16. Qi S, Chen H, Huang N, Li W, Liu G, Wang Y, et al. Early intervention improves clinical responses to house dust mite immunotherapy in allergic rhinitis patients. Int Arch Allergy Immunol. 2016; 171:234–240. PMID: 28049194.
Article17. Howarth P, Malling HJ, Molimard M, Devillier P. Analysis of allergen immunotherapy studies shows increased clinical efficacy in highly symptomatic patients. Allergy. 2012; 67:321–327. PMID: 22142377.
Article18. Ciprandi G, Tosca MA, Silvestri M. The practical role of serum allergen-specific IgE as potential biomarker for predicting responder to allergen immunotherapy. Expert Rev Clin Immunol. 2014; 10:321–324. PMID: 24450987.
Article19. Ciprandi G, Silvestri M. Serum specific IgE: a biomarker of response to allergen immunotherapy. J Investig Allergol Clin Immunol. 2014; 24:35–39.20. Tosca M, Silvestri M, Accogli A, Rossi GA, Ciprandi G. Serum-specific IgE and allergen immunotherapy in allergic children. Immunotherapy. 2014; 6:29–33. PMID: 24341881.
Article21. Olivieri M, Heinrich J, Schlünssen V, Antó JM, Forsberg B, Janson C, et al. The risk of respiratory symptoms on allergen exposure increases with increasing specific IgE levels. Allergy. 2016; 71:859–868. PMID: 26764559.
Article22. Corsico AG, De Amici M, Ronzoni V, Giunta V, Mennitti MC, Viscardi A, et al. Allergen-specific immunoglobulin E and allergic rhinitis severity. Allergy Rhinol (Providence). 2017; 8:1–4. PMID: 28381320.
Article23. Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009; 123:1103–1110. 1110.e1–1110.e4. PMID: 19356792.
Article24. Bousquet PJ, Castelli C, Daures JP, Heinrich J, Hooper R, Sunyer J, et al. Assessment of allergen sensitization in a general population-based survey (European Community Respiratory Health Survey I). Ann Epidemiol. 2010; 20:797–803. PMID: 20702109.
Article25. Passalacqua G. The use of single versus multiple antigens in specific allergen immunotherapy for allergic rhinitis: review of the evidence. Curr Opin Allergy Clin Immunol. 2014; 14:20–24. PMID: 24362238.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy
- Update of Sublingual Immunotherapy for Allergic Rhinitis
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Long-term Effects of Specific Allergen Immunotherapy Against House Dust Mites in Polysensitized Patients With Allergic Rhinitis
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex