Yonsei Med J.
2005 Dec;46(6):843-846.
A Case of Intolerance to Warfarin Dosing in an Intermediate Metabolizer of CYP2C9
- Affiliations
-
- 1Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
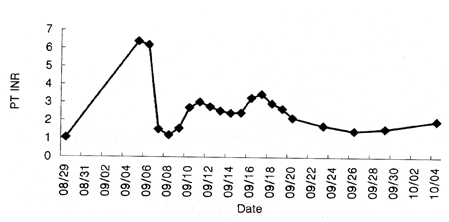
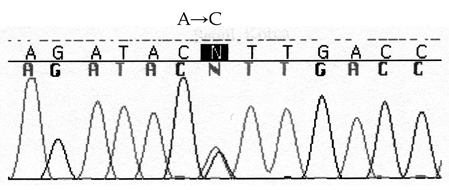
- We report a case of intolerance to warfarin dosing due to impaired drug metabolism in a patient heterozygous for the CYP2C9*3 allele. A 30-year-old woman with an artificial cardiac pacemaker was taking warfarin to prevent thromboembolism. This patient had an extremely elevated international normalized ratio (INR) of prothrombin time (PT) following standard doses of warfarin and experienced difficulties during the induction of anticoagulation. Genotyping for CYP2C9 revealed that this patient was an intermediate metabolizer with genotype CYP2C9*1/*3. This case suggests the clinical usefulness of pharmacogenetic testing for individualized dosage adjustments of warfarin.
MeSH Terms
Figure
Reference
-
1. Miners JO, Birkett DJ. Cytochrome P450C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998. 45:525–538.2. Takahashi H, Echizen H. Pharmacogenetics of CYP2C9 and interindividual variability in anticoagulant response to warfarin. Pharmacogenomics J. 2003. 3:202–214.3. Takahashi H, Echizen H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmacokinet. 2001. 40:587–603.4. Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1997. 353:717–719.5. Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997. 73:67–74.6. Daly AK, King BP. Pharmacogenetics of oral anticoagulants. Pharmacogenetics. 2003. 13:247–252.7. Higahi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FL, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002. 287:1690–1698.8. Tabrizi AR, Zehnbauer BA, Borecki IB, McGrath SD, Buchman TG, Freeman BD. The frequency and effects of cytochrome P450 (CYP) 2C9 polymorphisms in patients receiving warfarin. J Am Coll Surg. 2002. 194:267–273.9. Takahashi H, Kashima T, Nomizo Y, Muramoto N, Shimizu T, Nasu K, et al. Metabolism of warfarin enantiomers in Japanese patients with heart disease having different CYP2C9 and CYP2C19 genotypes. Clin Pharmacol Ther. 1998. 63:519–528.10. Scordo MG, Pengo V, Spina E, Dahl ML, Gusella M, Padrini R. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002. 72:702–710.11. Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002. 12:251–263.12. Stubbins MJ, Harries LW, Smith G, Tarbit MH, Wolf CR. Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics. 1996. 6:429–439.13. Bhasker CR, Miners JO, Coulter S, Birkett DJ. Allelic and functional variability of cytochrome P4502C9. Pharmacogenetics. 1997. 7:51–58.14. Ingelman-Sunberg M, Nebert DW, Daly AK. Human cytochrome P450 (CYP) genes. Available from: URL: http://www.imm.ki.se/CYPalleles/cyp2c9.htm.15. Yoon YR, Shon JH, Kim MK, Lim YC, Lee HR, Park JY, et al. Frequency of cytochrome P450 2C9 mutant alleles in a Korean population. Br J Clin Pharmacol. 2001. 51:277–280.16. Lee HC, Cho SY, Lee HJ, Kim CJ, Park JS, Chi JG. Warfarin-associated fetal intracranial hemorrhage: a Case Report. J Korean Med Sci. 2003. 18:764–767.17. Huh SH, Kim DI, Kim ES, Lee BB, Moon JY, Joh JH. Clinical and functional assessment after anticoagulant therapy of acute deep vein thrombosis involving the lower limb. Yonsei Med J. 2003. 44:686–693.18. Lee JH, Park JS, Chung SW, Sohn DK, Kim SH, Lee HS. Warfarin toxicity patients in the emergency department. J Korean Soc Emerg Med. 2003. 14:145–149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case Report of a Patient Carrying CYP2C9*3/4 Genotype with Extremely Low Warfarin Dose Requirement
- Development and Comparison of Warfarin Dosing Algorithms in Stroke Patients
- CYP2C9 Mutation Affecting the Individual Variability of Warfarin Dose Requirement
- Evaluation of the Verigene Warfarin Metabolism Nucleic Acid Test Kit for the Rapid Detection of CYP2C9 and VKORC1 Polymorphisms
- VKORC1 and CYP2C9 Genotype Variations in Relation to Warfarin Dosing in Korean Stroke Patients