Korean J Radiol.
2017 Apr;18(2):370-377. 10.3348/kjr.2017.18.2.370.
Longitudinal Intrinsic Brain Activity Changes in Cirrhotic Patients before and One Month after Liver Transplantation
- Affiliations
-
- 1Department of Radiology, Tianjin First Central Hospital, Tianjin 300192, China. shenwen66happy@163.com
- 2Department of Transplantation Surgery, Tianjin First Central Hospital, Tianjin 300192, China.
- 3School of Computer Science and Technology, Tianjin Key Laboratory of Cognitive Computing and Application, Tianjin University, Tianjin 300072, China.
- 4Department of Radiology, The Second Hospital of Tianjin Medical University, Tianjin 300211, China.
- KMID: 2427949
- DOI: http://doi.org/10.3348/kjr.2017.18.2.370
Abstract
OBJECTIVE
To evaluate the spontaneous brain activity alterations in liver transplantation (LT) recipients using resting-state functional MRI.
MATERIALS AND METHODS
Twenty cirrhotic patients as transplant candidates and 25 healthy controls (HCs) were included in this study. All patients repeated the MRI study one month after LT. Amplitude of low-frequency fluctuation (ALFF) values were compared between cirrhotic patients (both pre- and post-LT) and HCs as well as between the pre- and post-LT groups. The relationship between ALFF changes and venous blood ammonia levels and neuropsychological tests were investigated using Pearson's correlation analysis.
RESULTS
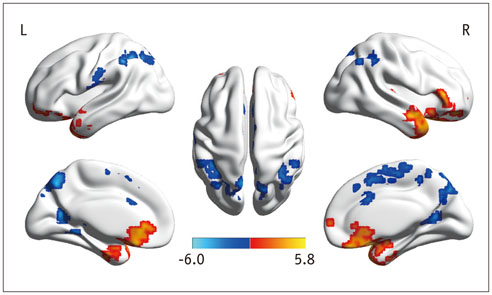
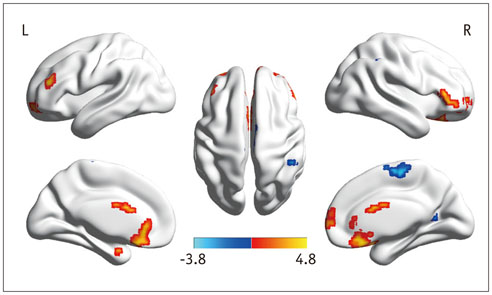
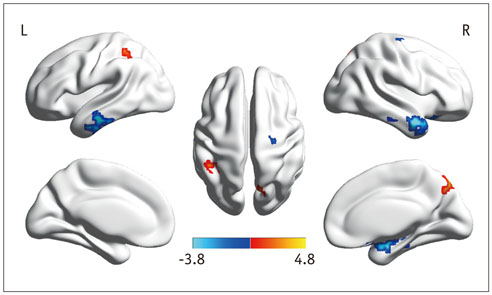
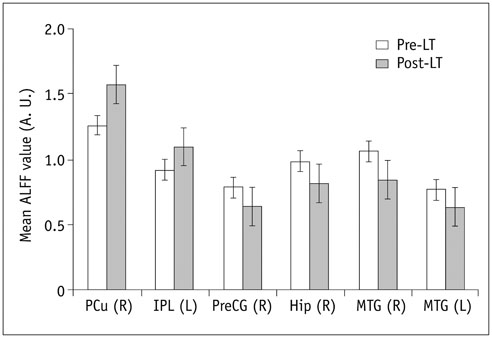
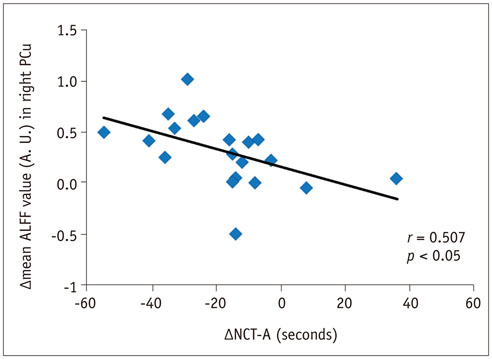
In the cirrhotic patients, decreased ALFF in the vision-related regions (left lingual gyrus and calcarine), sensorimotor-related regions (left postcentral gyrus and middle cingulate cortex), and the default-mode network (bilateral precuneus and left inferior parietal lobule) were restored, and the increased ALFF in the temporal and frontal lobe improved in the early period after LT. The ALFF decreases persisted in the right supplementary motor area, inferior parietal lobule, and calcarine. The ALFF changes in the right precuneus were negatively correlated with changes in number connection test-A scores (r = 0.507, p < 0.05).
CONCLUSION
LT improved spontaneous brain activity and the results for associated cognition tests. However, decreased ALFF in some areas persisted, and new-onset abnormal ALFF were possible, indicating that complete cognitive function recovery may need more time.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.
Reference
-
1. Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009; 50:2014–2021.2. Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011; 54:1030–1040.3. Cárdenas A, Ginès P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011; 60:412–421.4. Mechtcheriakov S, Graziadei IW, Mattedi M, Bodner T, Kugener A, Hinterhuber HH, et al. Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl. 2004; 10:77–83.5. Ortiz M, Córdoba J, Jacas C, Flavià M, Esteban R, Guardia J. Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol. 2006; 44:104–110.6. Pujol A, Graus F, Rimola A, Beltrán J, Garcia-Valdecasas JC, Navasa M, et al. Predictive factors of in-hospital CNS complications following liver transplantation. Neurology. 1994; 44:1226–1230.7. Guo WB, Liu F, Xue ZM, Yu Y, Ma CQ, Tan CL, et al. Abnormal neural activities in first-episode, treatment-naïve, short-illness-duration, and treatment-response patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. 2011; 135:326–331.8. Liu F, Hu M, Wang S, Guo W, Zhao J, Li J, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39:326–331.9. Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013; 70:845–851.10. Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014; 272:29–49.11. Ni L, Qi R, Zhang LJ, Zhong J, Zheng G, Wu X, et al. Brain regional homogeneity changes following transjugular intrahepatic portosystemic shunt in cirrhotic patients support cerebral adaptability theory--a resting-state functional MRI study. Eur J Radiol. 2014; 83:578–583.12. Ni L, Qi R, Zhang LJ, Zhong J, Zheng G, Zhang Z, et al. Altered regional homogeneity in the development of minimal hepatic encephalopathy: a resting-state functional MRI study. PLoS One. 2012; 7:e42016.13. Qi R, Zhang L, Wu S, Zhong J, Zhang Z, Zhong Y, et al. Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology. 2012; 264:187–195.14. Zhang L, Qi R, Wu S, Zhong J, Zhong Y, Zhang Z, et al. Brain default-mode network abnormalities in hepatic encephalopathy: a resting-state functional MRI study. Hum Brain Mapp. 2012; 33:1384–1392.15. Zhang LJ, Zheng G, Zhang L, Zhong J, Wu S, Qi R, et al. Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology. 2012; 265:528–536.16. Lin WC, Hsu TW, Chen CL, Lu CH, Chen HL, Cheng YF, et al. Reestablishing brain networks in patients without overt hepatic encephalopathy after liver transplantation. J Cereb Blood Flow Metab. 2014; 34:1877–1886.17. Zhang XD, Cheng Y, Poon CS, Qi R, Xu Q, Chen HJ, et al. Longand short-range functional connectivity density alteration in non-alcoholic cirrhotic patients one month after liver transplantation: a resting-state fMRI study. Brain Res. 2015; 1620:177–187.18. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007; 29:83–91.19. Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001; 34:768–773.20. Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010; 31:1851–1861.21. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004; 22:394–400.22. Chen HJ, Zhu XQ, Jiao Y, Li PC, Wang Y, Teng GJ. Abnormal baseline brain activity in low-grade hepatic encephalopathy: a resting-state fMRI study. J Neurol Sci. 2012; 318:140–145.23. Lv XF, Ye M, Han LJ, Zhang XL, Cai PQ, Jiang GH, et al. Abnormal baseline brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy revealed by resting-state functional MRI. Metab Brain Dis. 2013; 28:485–492.24. Qi R, Zhang LJ, Zhong J, Wu S, Zhang Z, Zhong Y, et al. Dynamic changes of intrinsic brain activity in cirrhotic patients after transjugular intrahepatic portosystemic shunt: a resting-state fMRI study. PLoS One. 2012; 7:e46681.25. Lee HW, Hong SB, Seo DW, Tae WS, Hong SC. Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology. 2000; 54:849–854.26. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006; 129(Pt 3):564–583.27. Luo S, Qi RF, Wen JQ, Zhong JH, Kong X, Liang X, et al. Abnormal intrinsic brain activity patterns in patients with end-stage renal disease undergoing peritoneal dialysis: a resting-state functional MR imaging study. Radiology. 2016; 278:181–189.28. Joebges EM, Heidemann M, Schimke N, Hecker H, Ennen JC, Weissenborn K. Bradykinesia in minimal hepatic encephalopathy is due to disturbances in movement initiation. J Hepatol. 2003; 38:273–280.29. Garcia-Martinez R, Rovira A, Alonso J, Jacas C, Simón-Talero M, Chavarria L, et al. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011; 17:38–46.30. Lin WC, Chou KH, Chen CL, Chen HL, Lu CH, Li SH, et al. Longitudinal brain white matter alterations in minimal hepatic encephalopathy before and after liver transplantation. PLoS One. 2014; 9:e105887.31. Mattarozzi K, Cretella L, Guarino M, Stracciari A. Minimal hepatic encephalopathy: follow-up 10 years after successful liver transplantation. Transplantation. 2012; 93:639–643.