J Pathol Transl Med.
2018 Nov;52(6):363-368. 10.4132/jptm.2018.09.18.
Immunohistochemistry of Janus Kinase 1 (JAK1) Expression in Vitiligo
- Affiliations
-
- 1Department of Pathology, Faculty of Medicine, Menoufia University, Shebein Elkom, Egypt. Asmaa_elsaidy@yahoo.com
- 2Department of Dermatology, Faculty of Medicine, Menoufia University, Shebein Elkom, Egypt.
- KMID: 2427518
- DOI: http://doi.org/10.4132/jptm.2018.09.18
Abstract
- BACKGROUND
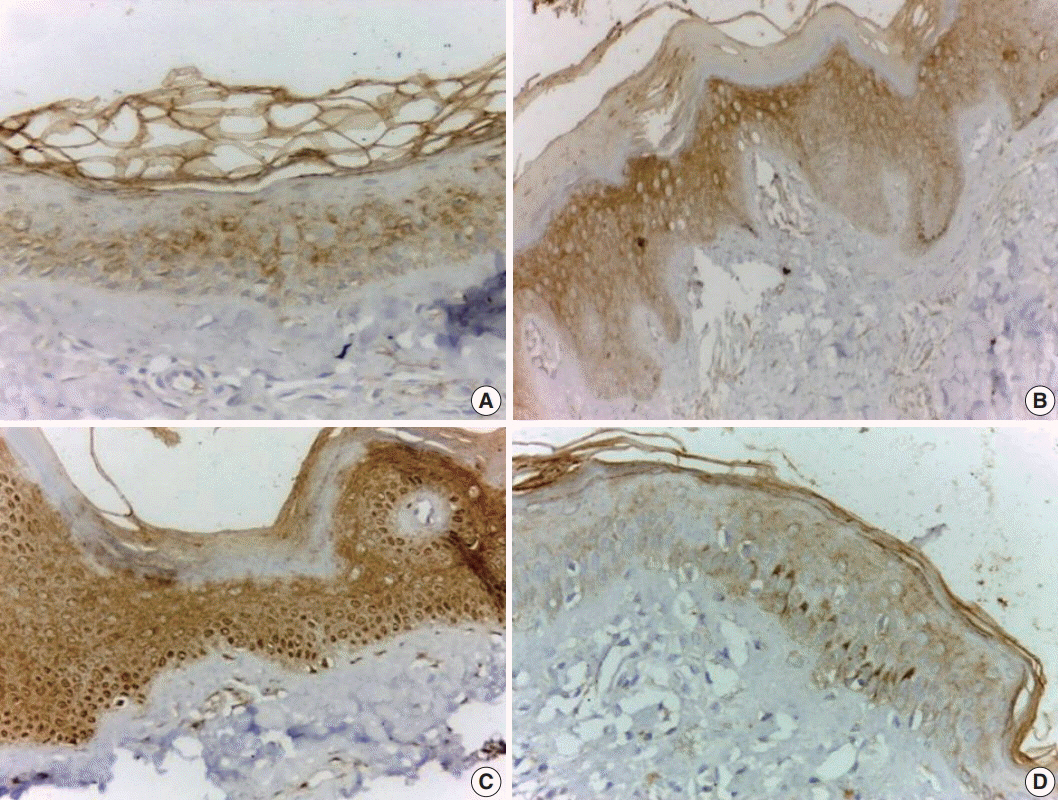
Vitiligo is a chronic autoimmune disease in which the destruction of melanocytes causes white spots on the affected skin. Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-signal transducer and activator of transcription pathway. The aim of the present study is to explore the possible role of JAK1 in the pathogenesis of vitiligo using immunohistochemical methods.
METHODS
The current study was conducted in a sample of 39 patients who presented with vitiligo and 22 healthy individuals who were age and sex matched as a control group. We used immunohistochemistry to evaluate JAK1 status (intensity and distribution) and assess the percentage of residual melanocytes using human melanoma black 45 (HMB45).
RESULTS
Intense and diffuse JAK1 expression was significantly more likely to indicate vitiliginous skin compared to normal skin (p < .001). Strong and diffuse JAK1 expression was associated with short disease duration, female sex, and lower percentage of melanocytes (detected by HMB45) (p < .05).
CONCLUSIONS
JAK1 may be involved in the pathogenesis of vitiligo, as indicated by intense and diffuse expression compared to control and association with lower percentage of melanocytes detected by HMB45 immunostaining.
Keyword
MeSH Terms
Figure
Reference
-
1. Bonotis K, Pantelis K, Karaoulanis S, et al. Investigation of factors associated with health-related quality of life and psychological distress in vitiligo. J Dtsch Dermatol Ges. 2016; 14:45–9.
Article2. Bhise SB, Nalawade AD, Wadhawa H. Role of protein tyrosine kinase inhibitors in cancer therapeutics. Indian J Biochem Biophys. 2004; 41:273–80.3. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000; 19:5662–79.
Article4. Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB. Evaluation of activatory and inhibitory natural killer cell receptors in non-segmental vitiligo: a flow cytometric study. J Eur Acad Dermatol Venereol. 2008; 22:970–6.
Article5. van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009; 129:2220–32.
Article6. Attwa E, Gamil H, Assaf M, Ghonemy S. Over-expression of tumor necrosis factor-alpha in vitiligo lesions after narrow-band UVB therapy: an immunohistochemical study. Arch Dermatol Res. 2012; 304:823–30.7. Klarquist J, Denman CJ, Hernandez C, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010; 23:276–86.
Article8. Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015; 151:1110–2.9. Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006; 72:315–21.
Article10. Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res. 2018; 310:39–46.11. Landry DA, Sormany F, Haché J, Roumaud P, Martin LJ. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol Cell Biochem. 2017; 433:79–95.
Article12. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2011; 36:292–7.
Article13. Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007; 8:247–56.
Article14. Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006; 126:1102–10.
Article15. Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005; 24:315–27.
Article16. Nakagawa R, Yoshida H, Asakawa M, et al. Pyridone 6, a pan-JAK inhibitor, ameliorates allergic skin inflammation of NC/Nga mice via suppression of Th2 and enhancement of Th17. J Immunol. 2011; 187:4611–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- Understanding of Pathomechanisms and Clinical Practice for Vitiligo
- Increased Expression of Tenascin on Photodamaged Vitiligo Skin
- Mutational Analysis of JAK1 Exons 10 and 13 in Non-small Cell Lung Cancers