Yonsei Med J.
2017 Mar;58(2):407-414. 10.3349/ymj.2017.58.2.407.
The Influences of Different Ratios of Biphasic Calcium Phosphate and Collagen Augmentation on Posterior Lumbar Spinal Fusion in Rat Model
- Affiliations
-
- 1Department of Neurosurgery, Spine and Spinal Cord Institute, Yonsei University College of Medicine, Seoul, Korea. kuhsu@yuhs.ac
- KMID: 2427131
- DOI: http://doi.org/10.3349/ymj.2017.58.2.407
Abstract
- PURPOSE
To determine the influence of different ratios of hydroxyapatite (HA)/beta tricalcium phosphate (β-TCP) and collagen augmentation for posterior lumbar fusion in a rat model.
MATERIALS AND METHODS
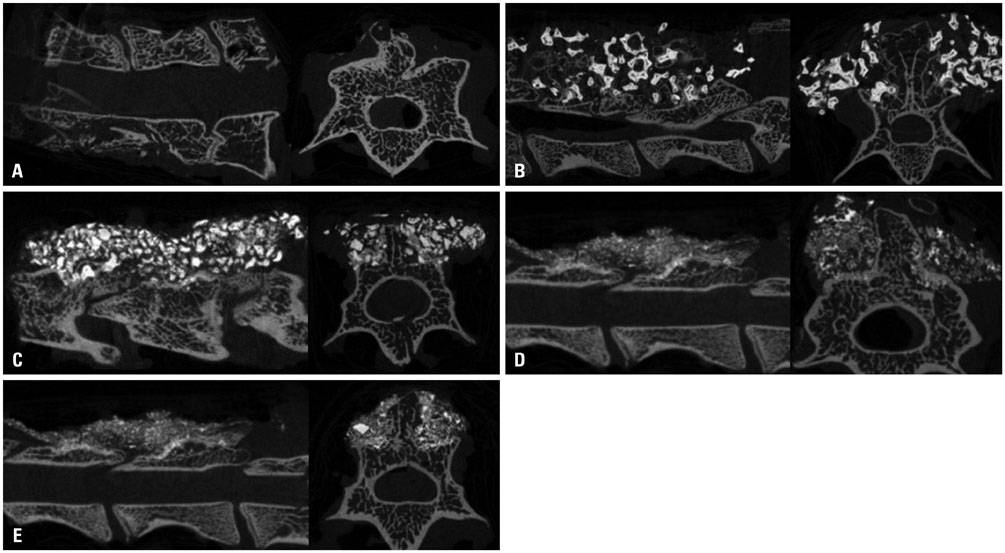
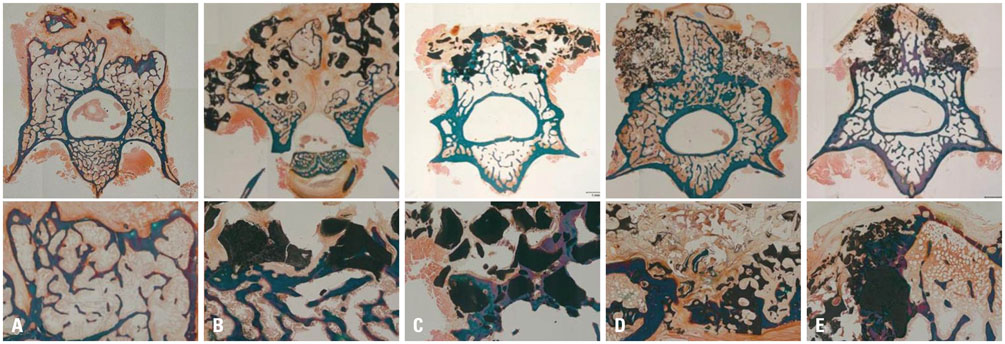
We generated a posterior lumbar fusion model in 50 rats and divided it into five groups of equal number as follows; 1) autologous bone graft as group A, 2) 70% HA+30% β-TCP as group B, 3) 70% HA+30% β-TCP+collagen as group C, 4) 30% HA+70% β-TCP as group D, and 5) 30% HA+70% β-TCP+collagen as group E. Rats were euthanized at 12 weeks after surgery and fusion was assessed by manual palpation, quantitative analysis using microCT and histology.
RESULTS
The score of manual palpation was significantly higher in group C than group E (3.1±1.1 vs. 1.8±0.8, p=0.033). However, in terms of microCT analysis, group D showed significantly higher scores than group B (5.5±0.8 vs. 3.1±1.1, p=0.021). According to quantitative volumetric analysis, 30% HA+70% β-TCP groups (group D and E) showed significantly reduced fusion mass at 12 weeks after surgery (123±14.2, 117±46.3 vs. 151±27.3, p=0.008, 0.003, respectively). Collagen augmentation groups revealed superior results in terms of both microCT score and histologic grade.
CONCLUSION
A 7:3 HA/β-TCP ratio with collagen augmentation rather than a 3:7 HA/β-TCP ratio could be a more favorable graft substitute for lumbar spinal fusion. There was positive role of collagen as an adjunct for spinal bone fusion process.
Keyword
MeSH Terms
Figure
Reference
-
1. Zurbriggen C, Markwalder TM, Wyss S. Long-term results in patients treated with posterior instrumentation and fusion for degenerative scoliosis of the lumbar spine. Acta Neurochir (Wien). 1999; 141:21–26.
Article2. Gertzbein SD, Hollopeter M, Hall SD. Analysis of circumferential lumbar fusion outcome in the treatment of degenerative disc disease of the lumbar spine. J Spinal Disord. 1998; 11:472–478.3. Rompe JD, Eysel P, Hopf C. Clinical efficacy of pedicle instrumentation and posterolateral fusion in the symptomatic degenerative lumbar spine. Eur Spine J. 1995; 4:231–237.
Article4. Herkowitz HN, Sidhu KS. Lumbar spine fusion in the treatment of degenerative conditions: current indications and recommendations. J Am Acad Orthop Surg. 1995; 3:123–135.
Article5. Flouzat-Lachaniette CH, Ghazanfari A, Bouthors C, Poignard A, Hernigou P, Allain J. Bone union rate with recombinant human bone morphogenic protein-2 versus autologous iliac bone in PEEK cages for anterior lumbar interbody fusion. Int Orthop. 2014; 38:2001–2007.
Article6. Kim DH, Rhim R, Li L, Martha J, Swaim BH, Banco RJ, et al. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J. 2009; 9:886–892.
Article7. Al-Sayyad MJ, Abdulmajeed TM. Fracture of the anterior iliac crest following autogenous bone grafting. Saudi Med J. 2006; 27:254–258.8. Pitzen T, Kränzlein K, Steudel WI, Strowitzki M. [Complaints and findings at the iliac crest donor site following anterior cervical fusion]. Zentralbl Neurochir. 2004; 65:7–12.9. Delawi D, Dhert WJ, Castelein RM, Verbout AJ, Oner FC. The incidence of donor site pain after bone graft harvesting from the posterior iliac crest may be overestimated: a study on spine fracture patients. Spine (Phila Pa 1976). 2007; 32:1865–1868.
Article10. Ito Z, Imagama S, Kanemura T, Hachiya Y, Miura Y, Kamiya M, et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (PLIF): a multicenter study. Eur Spine J. 2013; 22:1158–1163.
Article11. Lippman CR, Salehi SA, Liu JC, Ondra SL. Salvage technique of posterior iliac bolt placement in long-segment spinal constructs with a previous posterior iliac crest harvest: technical note. Neurosurgery. 2006; 58:1 Suppl. ONS–E178.
Article12. Emery SE, Hughes SS, Junglas WA, Herrington SJ, Pathria MN. The fate of anterior vertebral bone grafts in patients irradiated for neoplasm. Clin Orthop Relat Res. 1994; (300):207–212.
Article13. Taghavi CE, Lee KB, Keorochana G, Tzeng ST, Yoo JH, Wang JC. Bone morphogenetic protein-2 and bone marrow aspirate with allograft as alternatives to autograft in instrumented revision posterolateral lumbar spinal fusion: a minimum two-year follow-up study. Spine (Phila Pa 1976). 2010; 35:1144–1150.
Article14. Ajiboye RM, Hamamoto JT, Eckardt MA, Wang JC. Clinical and radiographic outcomes of concentrated bone marrow aspirate with allograft and demineralized bone matrix for posterolateral and interbody lumbar fusion in elderly patients. Eur Spine J. 2015; 24:2567–2572.
Article15. Virk S, Sandhu HS, Khan SN. Cost effectiveness analysis of graft options in spinal fusion surgery using a Markov model. J Spinal Disord Tech. 2012; 25:E204–E210.
Article16. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012; 132:1105–1110.
Article17. Thalgott JS, Giuffre JM, Fritts K, Timlin M, Klezl Z. Instrumented posterolateral lumbar fusion using coralline hydroxyapatite with or without demineralized bone matrix, as an adjunct to autologous bone. Spine J. 2001; 1:131–137.
Article18. Martin S, Daculsi G, Passuti N, Le Nihouannen JC, Lajat Y, Resche F. [Bone integration within calcium phosphate ceramic by creation of posterior inter-articular lumbar spine fusions in dogs]. Neurochirurgie. 1989; 35:209–215.19. Yi J, Lee GW, Nam WD, Han KY, Kim MH, Kang JW, et al. A prospective randomized clinical trial comparing bone union rate following anterior cervical discectomy and fusion using a polyetheretherketone cage: hydroxyapatite/B-tricalcium phosphate mixture versus hydroxyapatite/demineralized bone matrix mixture. Asian Spine J. 2015; 9:30–38.
Article20. Sugawara T, Itoh Y, Hirano Y, Higashiyama N, Mizoi K. β-tricalcium phosphate promotes bony fusion after anterior cervical discectomy and fusion using titanium cages. Spine (Phila Pa 1976). 2011; 36:E1509–E1514.
Article21. Cosar M, Ozer AF, Iplikcioglu AC, Oktenoglu T, Kosdere S, Sasani M, et al. The results of beta-tricalcium phosphate coated hydroxyapatite (beta-TCP/HA) grafts for interbody fusion after anterior cervical discectomy. J Spinal Disord Tech. 2008; 21:436–441.
Article22. Miron RJ, Sculean A, Shuang Y, Bosshardt DD, Gruber R, Buser D, et al. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin Oral Implants Res. 2016; 27:668–675.
Article23. Lobo SE, Glickman R, da Silva WN, Arinzeh TL, Kerkis I. Response of stem cells from different origins to biphasic calcium phosphate bioceramics. Cell Tissue Res. 2015; 361:477–495.
Article24. Calvo-Guirado JL, Ramírez-Fernández MP, Delgado-Ruíz RA, Maté-Sánchez JE, Velasquez P, de Aza PN. Influence of biphasic β-TCP with and without the use of collagen membranes on bone healing of surgically critical size defects. A radiological, histological, and histomorphometric study. Clin Oral Implants Res. 2014; 25:1228–1238.
Article25. Lim HC, Zhang ML, Lee JS, Jung UW, Choi SH. Effect of different hydroxyapatite: β-tricalcium phosphate ratios on the osteoconductivity of biphasic calcium phosphate in the rabbit sinus model. Int J Oral Maxillofac Implants. 2015; 30:65–72.
Article26. Ebrahimi M, Pripatnanont P, Suttapreyasri S, Monmaturapoj N. In vitro biocompatibility analysis of novel nano-biphasic calcium phosphate scaffolds in different composition ratios. J Biomed Mater Res B Appl Biomater. 2014; 102:52–61.
Article27. Nevins M, Nevins ML, Schupbach P, Kim SW, Lin Z, Kim DM. A prospective, randomized controlled preclinical trial to evaluate different formulations of biphasic calcium phosphate in combination with a hydroxyapatite collagen membrane to reconstruct deficient alveolar ridges. J Oral Implantol. 2013; 39:133–139.
Article28. Ebrahimi M, Pripatnanont P, Monmaturapoj N, Suttapreyasri S. Fabrication and characterization of novel nano hydroxyapatite/β-tricalcium phosphate scaffolds in three different composition ratios. J Biomed Mater Res A. 2012; 100:2260–2268.
Article29. Jensen SS, Bornstein MM, Dard M, Bosshardt DD, Buser D. Comparative study of biphasic calcium phosphates with different HA/TCP ratios in mandibular bone defects. A long-term histomorphometric study in minipigs. J Biomed Mater Res B Appl Biomater. 2009; 90:171–181.
Article30. Friedmann A, Dard M, Kleber BM, Bernimoulin JP, Bosshardt DD. Ridge augmentation and maxillary sinus grafting with a biphasic calcium phosphate: histologic and histomorphometric observations. Clin Oral Implants Res. 2009; 20:708–714.
Article31. LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med. 2003; 14:201–209.32. Bouler JM, LeGeros RZ, Daculsi G. Biphasic calcium phosphates: influence of three synthesis parameters on the HA/beta-TCP ratio. J Biomed Mater Res. 2000; 51:680–684.
Article33. Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992; 63:729–735.
Article34. Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004; (86-A):2243–2250.35. Biswas D, Bible JE, Whang PH, Miller CP, Jaw R, Miller S, et al. Augmented demineralized bone matrix: a potential alternative for posterolateral lumbar spinal fusion. Am J Orthop (Belle Mead NJ). 2010; 39:531–538.36. Kim JH, Park JY, Yi S, Kim KH, Kuh SU, Chin DK, et al. Anterior cervical discectomy and fusion alters whole-spine sagittal alignment. Yonsei Med J. 2015; 56:1060–1070.
Article37. Drespe IH, Polzhofer GK, Turner AS, Grauer JN. Animal models for spinal fusion. Spine J. 2005; 5:6 Suppl. 209S–216S.
Article38. Bostan B, Güneş T, Aşçı M, Sen C, Keles¸temur MH, Erdem M, et al. Simvastatin improves spinal fusion in rats. Acta Orthop Traumatol Turc. 2011; 45:270–275.
Article39. Maté-Sánchez de Val JE, Mazón P, Guirado JL, Ruiz RA, Ramírez Fernández MP, Negri B, et al. Comparison of three hydroxyapatite/ β-tricalcium phosphate/collagen ceramic scaffolds: an in vivo study. J Biomed Mater Res A. 2014; 102:1037–1046.
Article40. Yun PY, Kim YK, Jeong KI, Park JC, Choi YJ. Influence of bone morphogenetic protein and proportion of hydroxyapatite on new bone formation in biphasic calcium phosphate graft: two pilot studies in animal bony defect model. J Craniomaxillofac Surg. 2014; 42:1909–1917.
Article41. Eftekhari H, Farahpour MR, Rabiee SM. Histopathological evaluation of potential impact of β-tricalcium phosphate (HA+ β-TCP) granules on healing of segmental femur bone defect. Bratisl Lek Listy. 2015; 116:30–34.
Article42. Grimes JS, Bocklage TJ, Pitcher JD. Collagen and biphasic calcium phosphate bone graft in large osseous defects. Orthopedics. 2006; 29:145–148.
Article43. Muschler GF, Negami S, Hyodo A, Gaisser D, Easley K, Kambic H. Evaluation of collagen ceramic composite graft materials in a spinal fusion model. Clin Orthop Relat Res. 1996; 250–260.
Article44. Jégoux F, Goyenvalle E, Cognet R, Malard O, Moreau F, Daculsi G, et al. Mandibular segmental defect regenerated with macroporous biphasic calcium phosphate, collagen membrane, and bone marrow graft in dogs. Arch Otolaryngol Head Neck Surg. 2010; 136:971–978.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Union Rates of Autologous Bone Marrow, Local Autobone and Biphasic Calcium Phosphate Mixed Graft in Lumbar Posterolateral Fusion
- Biphasic Calcium Phosphate and Local Autobone Mixed Graft in Lumbar Posterolateral Fusion
- Comparison of the Bone Union Rates Using a Local Autobone and Bone Graft Substitute Mixed Graft in Lumbar Posterolateral Fusion
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- Late-term healing in an augmented sinus with different ratios of biphasic calcium phosphate: a pilot study using a rabbit sinus model