Characteristics of Adult Severe Refractory Asthma in Korea Analyzed From the Severe Asthma Registry
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Ewha Womans University, Seoul, Korea.
- 2Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. hjyoon@hanyang.ac.kr
- 3Department of Allergy and Respiratory Medicine, Konkuk University Medical Center, Seoul, Korea.
- 4Department of Pulmonary, Allergy and Critical Care Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Hallym University College of Medicine, Anyang, Korea.
- 6Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 7Division of Allergy and Clinical Immunology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. yscho@amc.seoul.kr
- 8Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea.
- 9Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 11Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 12Department of Internal Medicine, Chonnam National University Medical School & Hospital, Gwangju, Korea.
- 13Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 14Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2426784
- DOI: http://doi.org/10.4168/aair.2019.11.1.43
Abstract
- PURPOSE
Although mild to moderate asthma is much more common, the morbidity and mortality of severe asthma are much higher. This study was performed to identify and analyze the clinical characteristics of severe asthma in Korea.
METHODS
We registered patients with severe refractory asthma into the Severe Asthma Registry supported by the Severe Asthma Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology. Patients were enrolled since 2010 from the 15 university hospitals nationwide in Korea. Severe asthma was defined according to modified European Respiratory Society/American Thoracic Society criteria. Information on demographics, medical history, pulmonary function tests and skin prick tests was collected; the clinical characteristics of severe asthmatics were analyzed from the collected data.
RESULTS
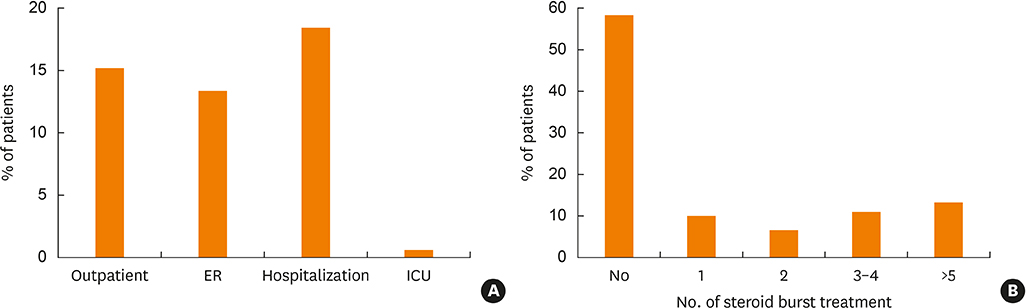
A total of 489 patients were enrolled with a mean age of 62.3; 45% are male. Sixty percent of patients received Global Initiative for Asthma step 4 treatment, and 30% received step 5 treatment. The most common comorbidities were allergic rhinitis (58.7%). Aspirin hypersensitivity was observed in 14.0%. Approximately half (53.9%) are non-smokers. Atopy was proven in 38.5% of the patients. Regarding asthma medications, inhaled corticosteroids and long-acting β-agonist combination inhalers were most commonly prescribed (96.5%), followed by leukotriene antagonists (71.0%). A recombinant anti-immunoglobulin E monoclonal antibody (omalizumab) has been used in 1.8% of the patients. The mean forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and FEV1/FVC were 78.7%, 67.5% and 67.9% of predicted values, respectively. The mean Asthma Control Test and quality of life questionnaire scores were 16.5 out of 25 and 59.5 out of 85, respectively.
CONCLUSIONS
The baseline characteristics of severe asthma patients in the Korea Severe Asthma Registry were analyzed and reported for the first time. With this cohort, further prospective studies should be performed to search for ways to improve management of severe refractory asthma.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones
Adult*
Allergy and Immunology
Aspirin
Asthma*
Cohort Studies
Comorbidity
Demography
Forced Expiratory Volume
Hospitals, University
Humans
Hypersensitivity
Korea*
Leukotriene Antagonists
Male
Mortality
Nebulizers and Vaporizers
Prospective Studies
Quality of Life
Respiratory Function Tests
Rhinitis, Allergic
Skin
Vital Capacity
Adrenal Cortex Hormones
Aspirin
Leukotriene Antagonists
Figure
Cited by 6 articles
-
Future Risks in Patients With Severe Asthma
Woo-Jung Song, Ji-Hyang Lee, Yewon Kang, Woo Joung Joung, Kian Fan Chung
Allergy Asthma Immunol Res. 2019;11(6):763-778. doi: 10.4168/aair.2019.11.6.763.Inhaled Corticosteroids in Asthma and the Risk of Pneumonia
Min-Hye Kim, Chin Kook Rhee, Ji-Su Shim, So Young Park, Kwang Ha Yoo, Bo Yeon Kim, Hye Won Bae, Yun Su Sim, Jung Hyun Chang, Young-Joo Cho, Jin Hwa Lee
Allergy Asthma Immunol Res. 2019;11(6):795-805. doi: 10.4168/aair.2019.11.6.795.Is Trabecular Bone Score a More Sensitive Marker for Osteoporosis in Asthmatics?
Cheol-Woo Kim
Allergy Asthma Immunol Res. 2019;11(3):302-305. doi: 10.4168/aair.2019.11.3.302.Efficacy and Safety of a Pressurized Metered-Dose Inhaler in Older Asthmatics: Comparison to a Dry Powder Inhaler in a 12-Week Randomized Trial
Seong-Dae Woo, Young-Min Ye, Youngsoo Lee, So-Hee Lee, Yoo Seob Shin, Joo Hun Park, Hyunna Choi, Hyun-Young Lee, Hyun-Jung Shin, Hae-Sim Park
Allergy Asthma Immunol Res. 2020;12(3):454-466. doi: 10.4168/aair.2020.12.3.454.Increasing Prevalence and Mortality of Asthma With Age in Korea, 2002–2015: A Nationwide, Population-Based Study
Eunyoung Lee, Anhye Kim, Young-Min Ye, Sang-Eun Choi, Hae-Sim Park
Allergy Asthma Immunol Res. 2020;12(3):467-484. doi: 10.4168/aair.2020.12.3.467.Phenotype of Asthma-COPD Overlap in COPD and Severe Asthma Cohorts
Hyonsoo Joo, So-Young Park, So Young Park, Seo Young Park, Sang-Heon Kim, You Sook Cho, Kwang Ha Yoo, Ki Suck Jung, Chin Kook Rhee
J Korean Med Sci. 2022;37(30):e236. doi: 10.3346/jkms.2022.37.e236.
Reference
-
1. Martinez FD, Vercelli D. Asthma. Lancet. 2013; 382:1360–1372.
Article2. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–534.
Article3. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43:343–373.4. Barnes PJ, Woolcock AJ. Difficult asthma. Eur Respir J. 1998; 12:1209–1218.
Article5. Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000; 106:1033–1042.
Article6. O'Byrne PM, Naji N, Gauvreau GM. Severe asthma: future treatments. Clin Exp Allergy. 2012; 42:706–711.7. American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000; 162:2341–2351.8. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015; 135:896–902.
Article9. Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, et al. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. Eur Respir J. 1999; 13:1198–1208.10. Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007; 119:1337–1348.
Article11. Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI). Thorax. 2011; 66:910–917.
Article12. Lang DM. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc. 2015; 36:418–424.
Article13. Kim SH, Kim TW, Kwon JW, Kang HR, Lee YW, Kim TB, et al. Economic costs for adult asthmatics according to severity and control status in Korean tertiary hospitals. J Asthma. 2012; 49:303–309.
Article14. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41:1308–1314.
Article15. Kim TB, Park CS, Bae YJ, Cho YS, Moon HB. COREA Study Group. Factors associated with severity and exacerbation of asthma: a baseline analysis of the cohort for reality and evolution of adult asthma in Korea (COREA). Ann Allergy Asthma Immunol. 2009; 103:311–317.
Article16. Kim MA, Shin SW, Park JS, Uh ST, Chang HS, Bae DJ, et al. Clinical characteristics of exacerbation-prone adult asthmatics identified by cluster analysis. Allergy Asthma Immunol Res. 2017; 9:483–490.
Article17. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.
Article18. Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015; 46:1308–1321.19. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017; 9:386–393.
Article20. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention [Internet]. place unknown: Global Initiative for Asthma;2017. cited 2017 Feb 28. Available from: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/.21. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65.22. Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, et al. Correlation between the Korean version of Asthma Control Test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008; 23:621–627.
Article23. Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med. 2014; 108:1723–1732.
Article24. Yune S, Lee JY, Choi DC, Lee BJ. Fractional exhaled nitric oxide: comparison between portable devices and correlation with sputum eosinophils. Allergy Asthma Immunol Res. 2015; 7:404–408.
Article25. Siroux V, Basagaña X, Boudier A, Pin I, Garcia-Aymerich J, Vesin A, et al. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J. 2011; 38:310–317.
Article26. Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006; 117:549–556.
Article27. Menzella F, Galeone C, Formisano D, Castagnetti C, Ruggiero P, Simonazzi A, et al. Real-life efficacy of omalizumab after 9 years of follow-up. Allergy Asthma Immunol Res. 2017; 9:368–372.
Article28. Li J, Kang J, Wang C, Yang J, Wang L, Kottakis I, et al. China Omalizumab Study Group. Omalizumab improves quality of life and asthma control in chinese patients with moderate to severe asthma: a randomized phase iii study. Allergy Asthma Immunol Res. 2016; 8:319–328.
Article29. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.
Article