Isotretinoin exposure in pregnant women in Korea
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Cheil General Hospital and Women's Healthcare Center, Dankook University School of Medicine, Seoul, Korea. hanjungyeol055@gmail.com
- 2Department of Obstetrics and Gynecology, Ilsin Christian Hospital, Busan, Korea.
- 3Department of Obstetrics and Gynecology, Miz Women's Hospital, Daejeon, Korea.
- 4Department of Obstetrics and Gynecology, Mam's Women's Hospital, Ulsan, Korea.
- 5Department of Obstetrics and Gynecology, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 6Department of Obstetrics and Gynecology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2426007
- DOI: http://doi.org/10.5468/ogs.2018.61.6.649
Abstract
OBJECTIVE
Isotretinoin is a notorious teratogen otherwise used for the treatment of acne vulgaris. Some countries, including those in North America and the European Union, implemented the pregnancy prevention program (PPP); however, no PPP has yet been established in South Korea. So the aim of this study was to evaluate the rate of pregnant women exposed to isotretinoin among the callers of the Korean Mother Safe Counseling Center.
METHODS
This is a prospective cohort study. We evaluated the demographic characteristics, obstetric history, and isotretinoin exposure of pregnant women based on the mother safe registry from April 2010 to July 2016.
RESULTS
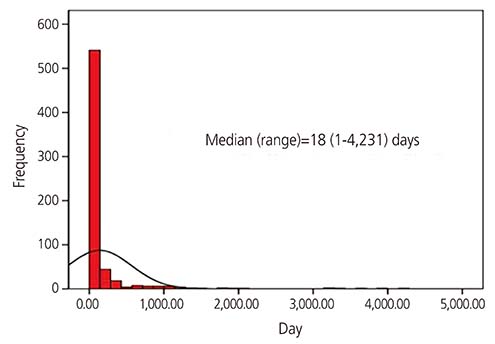
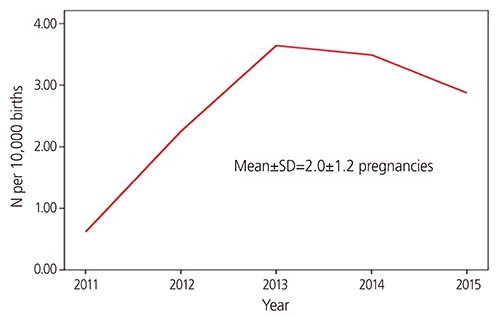
Among 22,374 callers, 650 (2.9%) pregnant women were exposed to isotretinoin. The mean age was 29.0±4.4 years in the isotretinoin-exposed group and 32.0±4.2 years in the unexposed group (P < 0.001). Moreover, the incidence of pregnancies within 30 days after isotretinoin discontinuation or during isotretinoin intake was 78.9% (513/650). The median duration of isotretinoin exposure was 18 (1-4,231) days. Furthermore, from 2011 to 2015, the incidence of isotretinoin exposure was 2.9±1.2 pregnancies per 10,000 births in South Korea.
CONCLUSION
Approximately 80% of pregnant women are exposed to isotretinoin within the recommended 30 days of contraception or during pregnancy. Therefore, the PPP has to be established in South Korea.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Korean Mothersafe Center 10th Anniversary: Outcome and Future Prospects
Jung Yeol Han, Hyun Kyong Ahn, June Seek Choi, Gye Jeong Yeom, So-Young Lee, Yoon Ha Kim, Dal Soo Hong, Seong Yeon Hong, Jeong Sup Yun, Hye Jin Jung, Hye Ji Jeon, Sung Hong Joo, Anna Choi, Eui Shik Jeong
J Korean Soc Matern Child Health. 2019;23(4):209-219. doi: 10.21896/jksmch.2019.23.4.209.Non-compliance with pregnancy prevention recommendations for isotretinoin in Korea between 2019–2020
Eun Jeong Choi, Jung Yeol Han
Obstet Gynecol Sci. 2021;64(2):201-208. doi: 10.5468/ogs.20247.The rates of major malformations after gestational exposure to isotretinoin: a systematic review and meta-analysis
Eun Jeong Choi, NaeRy Kim, Ho-Seok Kwak, Hae Ji Han, Kyoung-Chul Chun, Young-Ah Kim, Jae-Whoan Koh, Jung Yeol Han, Sung Hong Joo, Ji Sung Lee, Gideon Koren
Obstet Gynecol Sci. 2021;64(4):364-373. doi: 10.5468/ogs.20373.Pregnancy and neonatal outcomes after periconceptional exposure to isotretinoin in Koreans
Eun-Hwan Cha, NaeRy Kim, Ho-Seok Kwak, Hae Ji Han, Sung Hong Joo, June-Seek Choi, Kyoung-Chul Chun, Young-Ah Kim, Jae-Whoan Koh, Jung Yeol Han
Obstet Gynecol Sci. 2022;65(2):166-175. doi: 10.5468/ogs.21354.
Reference
-
1. Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009; 1:162–169.
Article2. Korea Pharmaceutical Information Center. Isotretinoin information [Internet]. Seoul: Korea Pharmaceutical Information Center;c2018. cited 2018 Feb 19. Available from: http://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AHHHHH0304.3. Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin for acne from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006; 62:667–674.
Article4. Honein MA, Paulozzi LJ, Erickson JD. Continued occurrence of Accutane-exposed pregnancies. Teratology. 2001; 64:142–147.
Article5. Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985; 313:837–841.
Article6. Stern RS. When a uniquely effective drug is teratogenic. The case of isotretinoin. N Engl J Med. 1989; 320:1007–1009.
Article7. Health Insurance Review & Assessment service. Health insurance statistical information [Internet]. Wonju: Health Insurance Review & Assessment Service;c2017. cited 2018 Feb 19. Available from: http://opendata.hira.or.kr/op/opc/olapGnlInfo.do.8. Han JY, Nava-Ocampo AA, Koren G. Unintended pregnancies and exposure to potential human teratogens. Birth Defects Res A Clin Mol Teratol. 2005; 73:245–248.
Article9. Dathe K, Schaefer C. Drug safety in pregnancy: the German Embryotox institute. Eur J Clin Pharmacol. 2018; 74:171–179.
Article10. Abroms L, Maibach E, Lyon-Daniel K, Feldman SR. What is the best approach to reducing birth defects associated with isotretinoin? PLoS Med. 2006; 3:e483.
Article11. iPLEDGE. iPLEDGE information [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;c2016. cited 2018 Feb 19. Available from: https://www.ipledgeprogram.com.12. Zomerdijk IM, Ruiter R, Houweling LM, Herings RM, Sturkenboom MC, Straus SM, et al. Isotretinoin exposure during pregnancy: a population-based study in the Netherlands. BMJ Open. 2014; 4:e005602.
Article13. Statistics Korea. Newborn statistical information [Internet]. Daejeon: Statistics Korea;c2014. cited 2018 Feb 19. Available from: http://m.kosis.kr/mobService/jipyolist/ModDataD.do?parmTitleYear=0&parmMainJipyo=601&parmPrdSe=&parmPrdDe=&parmAreaType=1&parmTypeGubun=D&preCode=A&preListNm=%EC%9D%B8%EA%B5%AC%EA%B0%80%EA%B5%AC&rn=3.14. Mitchell AA, Van Bennekom CM, Louik C. A pregnancy-prevention program in women of childbearing age receiving isotretinoin. N Engl J Med. 1995; 333:101–106.
Article15. Yook JH, Han JY, Choi JS, Ahn HK, Lee SW, Kim MY, et al. Pregnancy outcomes and factors associated with voluntary pregnancy termination in women who had been treated for acne with isotretinoin. Clin Toxicol (Phila). 2012; 50:896–901.
Article16. Bérard A, Azoulay L, Koren G, Blais L, Perreault S, Oraichi D. Isotretinoin, pregnancies, abortions and birth defects: a population-based perspective. Br J Clin Pharmacol. 2007; 63:196–205.
Article17. Stathakis V, Kilkenny M, Marks R. Descriptive epidemiology of acne vulgaris in the community. Australas J Dermatol. 1997; 38:115–123.
Article18. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013; 168:474–485.
Article19. Health Insurance Review & Assessment Service. Health insurance statistical information [Internet]. Wonju: Health Insurance Review & Assessment Service;c2017. cited 2018 Feb 19. Available from: http://opendata.hira.or.kr/home.do.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Suspected Isotretinoin-Induced Malformation in a Baby of a Mother Who Became Pregnant One Month after Discontinuation of the Drug
- Pregnancy and neonatal outcomes after periconceptional exposure to isotretinoin in Koreans
- Usefulness and limitations of Chat GPT in getting information on teratogenic drugs exposed in pregnancy
- The rates of major malformations after gestational exposure to isotretinoin: a systematic review and meta-analysis
- A Case of Confluent and Reticulated Papillomatosis Treated with Oral Isotretinoin