Obstet Gynecol Sci.
2022 Mar;65(2):166-175. 10.5468/ogs.21354.
Pregnancy and neonatal outcomes after periconceptional exposure to isotretinoin in Koreans
- Affiliations
-
- 1Korean Mothersafe Counselling Center, Pregnancy & Breastfeeding Medicines Information Center, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Inje University Ilsan Paik Hospital, Goyang, Korea
- 3Department of Obstetrics and Gynecology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- 4Department of Obstetrics and Gynecology, National Medical Center, Seoul, Korea
- 5Department of Obstetrics and Gynecology, CHA Gangnam Medical Center, CHA University, Seoul, Korea
- KMID: 2527268
- DOI: http://doi.org/10.5468/ogs.21354
Abstract
Objective
Isotretinoin should not be used during pregnancy because of the risk of birth defects. Most pregnant women exposed to isotretinoin choose voluntary pregnancy termination due to concerns about birth defects. However, birth outcome data supporting the termination of pregnancy are lacking. This study aimed to evaluate pregnancy and neonatal outcomes after periconception exposure to isotretinoin.
Methods
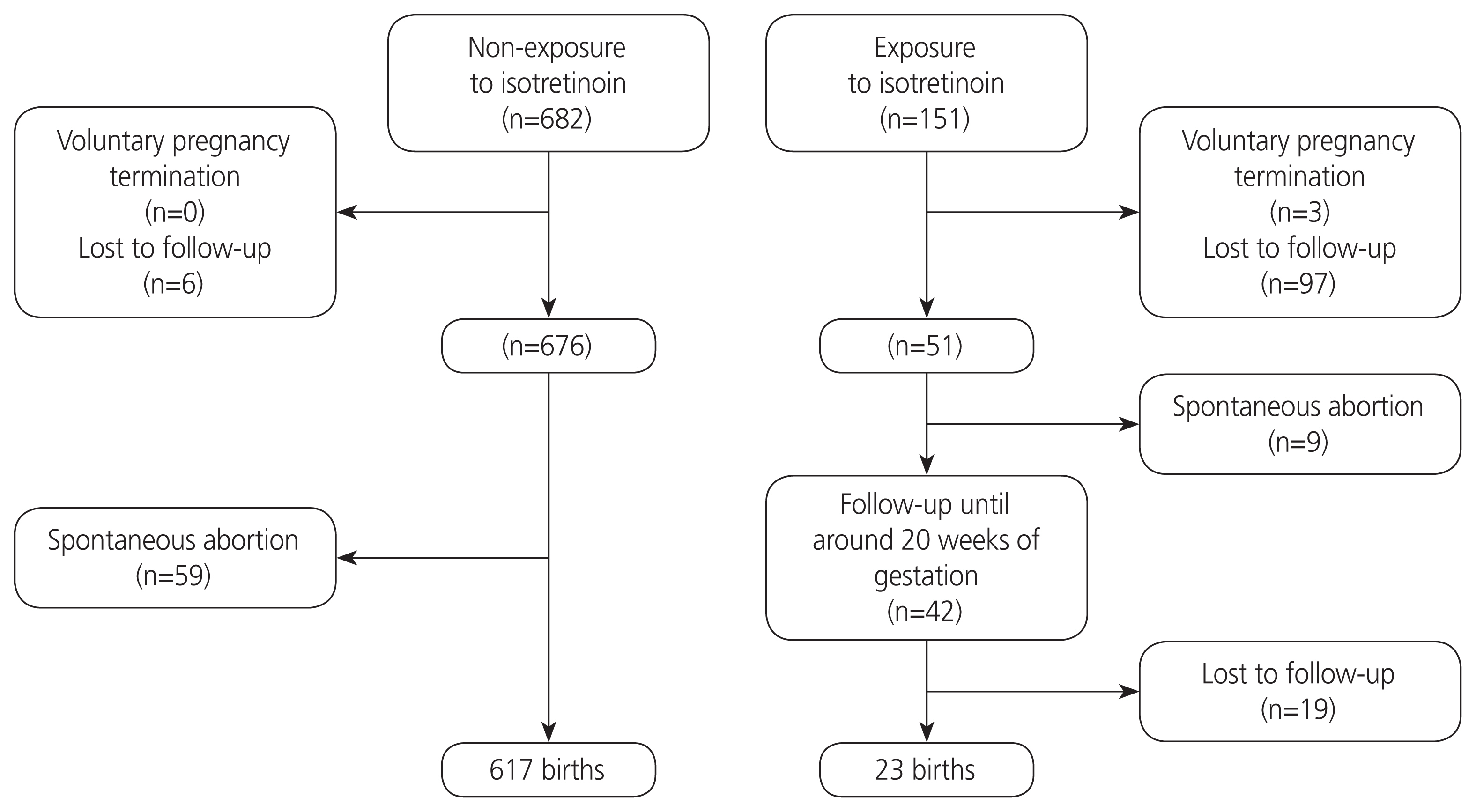
This was a prospective cohort study. We evaluated pregnancy and neonatal outcomes after exposure to isotretinoin in 151 pregnant women. Among 1,026 callers at the Korean Teratology Information Service from 2001 to 2017 exposed to isotretinoin during the periconception period, 151 pregnant women who received counseling on teratogenic risk after visiting the clinic were included.
Results
Among the 151 participants who visited the clinic, only 42 were evaluated using ultrasonography until approximately 20 weeks of gestation. Ultimately, 23 patients were included in the study. The average gestation period during the last exposure to the drug was 2 weeks, and the average daily exposure dose was 12 mg. There were two cases of major birth defects in the exposure group. Spontaneous abortion rates were 17.7% and 8.7% in the exposure and nonexposure groups, respectively (P=0.035). There was no significant difference between the exposure and non-exposure groups in terms of pregnancy and neonatal outcomes.
Conclusion
There was no significant difference in pregnancy and neonatal outcomes, including birth defects, between the exposure and non-exposure groups. Further studies with larger sample sizes are required to validate our findings.
Figure
Cited by 1 articles
-
Usefulness and limitations of Chat GPT in getting information on teratogenic drugs exposed in pregnancy
Jung Yeol Han
Obstet Gynecol Sci. 2025;68(1):1-8. doi: 10.5468/ogs.24231.
Reference
-
References
1. Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009; 1:162–9.
Article2. Korea Pharmaceutical Information Center. Isotretinoin [Internet]. Seoul (KR): Korea Pharmaceutical Information Center;c2000. [cited 2021 Aug 17]. Available from: http://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AHHHHH0355 .3. Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985; 313:837–41.
Article4. Zomerdijk IM, Ruiter R, Houweling LM, Herings RM, Sturkenboom MC, Straus SM, et al. Isotretinoin exposure during pregnancy: a population-based study in the Netherlands. BMJ Open. 2014; 4:e005602.
Article5. Kim NR, Yoon SR, Choi JS, Ahn HK, Lee SY, Hong DS, et al. Isotretinoin exposure in pregnant women in Korea. Obstet Gynecol Sci. 2018; 61:649–54.
Article6. Yook JH, Han JY, Choi JS, Ahn HK, Lee SW, Kim MY, et al. Pregnancy outcomes and factors associated with voluntary pregnancy termination in women who had been treated for acne with isotretinoin. Clin Toxicol (Phila). 2012; 50:896–901.
Article7. Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol. 2015; 126:1075–84.8. Dai WS, LaBraico JM, Stern RS. Epidemiology of isotretinoin exposure during pregnancy. J Am Acad Dermatol. 1992; 26:599–606.
Article9. Henry D, Dormuth C, Winquist B, Carney G, Bugden S, Teare G, et al. Occurrence of pregnancy and pregnancy outcomes during isotretinoin therapy. CMAJ. 2016; 188:723–30.
Article10. Schaefer C, Meister R, Weber-Schoendorfer C. Isotretinoin exposure and pregnancy outcome: an observational study of the berlin institute for clinical teratology and drug risk assessment in pregnancy. Arch Gynecol Obstet. 2010; 281:221–7.
Article11. Davis WL, Jacoby BH, Farmer GR, Cooper OJ. Changes in cytosolic calcium, bleb formation, and cell death in neural crest cells treated with isotretinoin and 4-oxo-isotretinoin. J Craniofac Genet Dev Biol. 1991; 11:105–18.12. Choi EJ, Kim N, Kwak HS, Han HJ, Chun KC, Kim YA, et al. The rates of major malformations after gestational exposure to isotretinoin: a systematic review and meta-analysis. Obstet Gynecol Sci. 2021; 64:364–73.
Article13. Loureiro KD, Kao KK, Jones KL, Alvarado S, Chavez C, Dick L, et al. Minor malformations characteristic of the retinoic acid embryopathy and other birth outcomes in children of women exposed to topical tretinoin during early pregnancy. Am J Med Genet A. 2005; 136:117–21.
Article14. Choi EJ, Han JY. Non-compliance with pregnancy prevention recommendations for isotretinoin in Korea between 2019–2020. Obstet Gynecol Sci. 2021; 64:201–8.
Article15. iPLEDGE. Committed to pregnancy prevention [Internet]. Morgantown (NY): iPLEDGE;c2021. [cited 2021 Aug 17]. Available from: https://www.ipledgeprogram.com/#Main/PublicHame .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Impetigo Herpetiformis Treated with Isotretinoin
- Isotretinoin exposure in pregnant women in Korea
- A Case of Suspected Isotretinoin-Induced Malformation in a Baby of a Mother Who Became Pregnant One Month after Discontinuation of the Drug
- Non-compliance with pregnancy prevention recommendations for isotretinoin in Korea between 2019-2020
- A Case of Confluent and Reticulated Papillomatosis Treated with Oral Isotretinoin