Yonsei Med J.
2009 Jun;50(3):445-447. 10.3349/ymj.2009.50.3.445.
A Case of Suspected Isotretinoin-Induced Malformation in a Baby of a Mother Who Became Pregnant One Month after Discontinuation of the Drug
- Affiliations
-
- 1Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. minspak@yuhs.ac
- 2Department of Radiology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1758576
- DOI: http://doi.org/10.3349/ymj.2009.50.3.445
Abstract
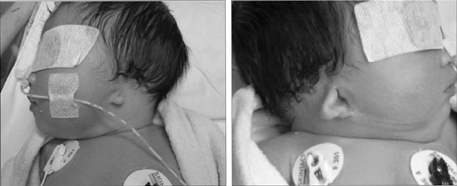
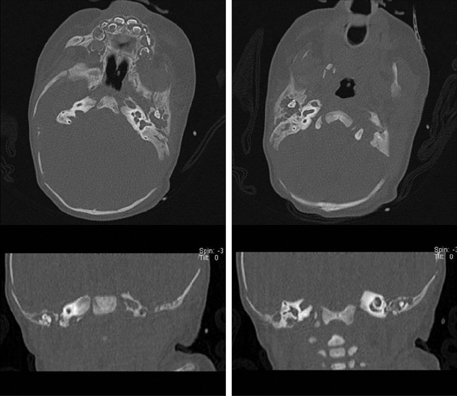
- Isotretinoin is a known human teratogen that can cause multiple malformations. At present, women who conceive one cycle after discontinuing isotretinoin are told that their teratogenic risk is not higher than baseline. We present a case of both ear malformation in a newborn whose mother had taken isotretinoin for 2 years until one month prior to the time when she became pregnant. We suggest that further studies of pharmacokinetics and malformation of isotreinoin are needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Accutane-exposed pregnancies--California, 1999. MMWR Morb Mortal Wkly Rep. 2000. 49:28–31.2. Brinker A, Kornegay C, Nourjah P. Trends in adherence to a revised risk management program designed to decrease or eliminate isotretinoin-exposed pregnancies: evaluation of the accutane SMART program. Arch Dermatol. 2005. 141:563–569.3. Mitchell AA, Van Bennekom CM, Louik C. A pregnancy-prevention program in women of childbearing age receiving isotretinoin. N Engl J Med. 1995. 333:101–106.
Article4. Vucinovic M, Roje D, Vucinovic Z, Capkun V, Bucat M, Banovic I. Maternal and neonatal effects of substance abuse during pregnancy: our ten-year experience. Yonsei Med J. 2008. 49:705–713.
Article5. Honein MA, Lindstrom JA, Kweder SL. Can we ensure the safe use of known human teratogens?: the iPLEDGE test case. Drug Saf. 2007. 30:5–15.6. Webster WS, Johnston MC, Lammer EJ, Sulik KK. Isotretinoin embryopathy and the cranial neural crest: an in vivo and in vitro study. J Craniofac Genet Dev Biol. 1986. 6:211–222.7. Suutarla S, Rautio J, Ritvanen A, Ala-Mello S, Jero J, Klockars T. Microtia in Finland: comparison of characteristics in different populations. Int J Pediatr Otorhinolaryngol. 2007. 71:1211–1217.
Article8. Hersh JH, Danhauer DE, Hand ME, Weisskopf B. Retinoic acid embryopathy: timing of exposure and effects on fetal development. JAMA. 1985. 254:909–910.
Article9. Dai WS, Hsu MA, Itri LM. Safety of pregnancy after discontinuation of isotretinoin. Arch Dermatol. 1989. 125:362–365.
Article10. Nulman I, Berkovitch M, Klein J, Pastuszak A, Lester RS, Shear N, et al. Steady-state pharmacokinetics of isotretinoin and its 4-oxo metabolite: implications for fetal safety. J Clin Pharmacol. 1998. 38:926–930.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isotretinoin exposure in pregnant women in Korea
- Usefulness and limitations of Chat GPT in getting information on teratogenic drugs exposed in pregnancy
- Response Guidelines for Newborn Infants Born to Mothers with Suspected or Confirmed Coronavirus Disease 2019
- A Case of Confluent and Reticulated Papillomatosis Treated with Oral Isotretinoin
- Drugs and Breastfeeding