Intest Res.
2016 Apr;14(2):152-163. 10.5217/ir.2016.14.2.152.
Adalimumab induction and maintenance therapy achieve clinical remission and response in Chinese patients with Crohn's disease
- Affiliations
-
- 1Xijing Hospital of the Fourth Military Medical University, Xi'an, China. kaicwu@fmmu.edu.cn
- 2Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
- 3The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- 4The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
- 5Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
- 6The Military General Hospital of Beijing, PLA, Beijing, China.
- 7St. Vincent's Hospital and University of Melbourne, Melbourne, Australia.
- 8Imperial College, London, UK.
- 9Translational Gastroenterology Unit, Oxford, UK.
- 10AbbVie, North Chicago, Illinois, USA.
- KMID: 2425288
- DOI: http://doi.org/10.5217/ir.2016.14.2.152
Abstract
- BACKGROUND/AIMS
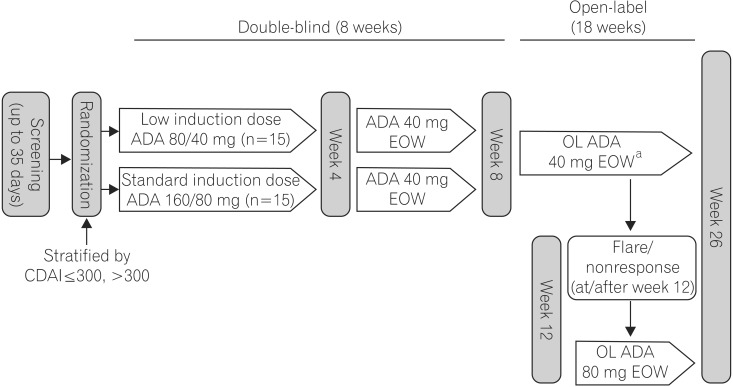
This was a Phase 2 study (NCT02015793) to evaluate the pharmacokinetics, safety, and efficacy of adalimumab in Chinese patients with Crohn's disease (CD).
METHODS
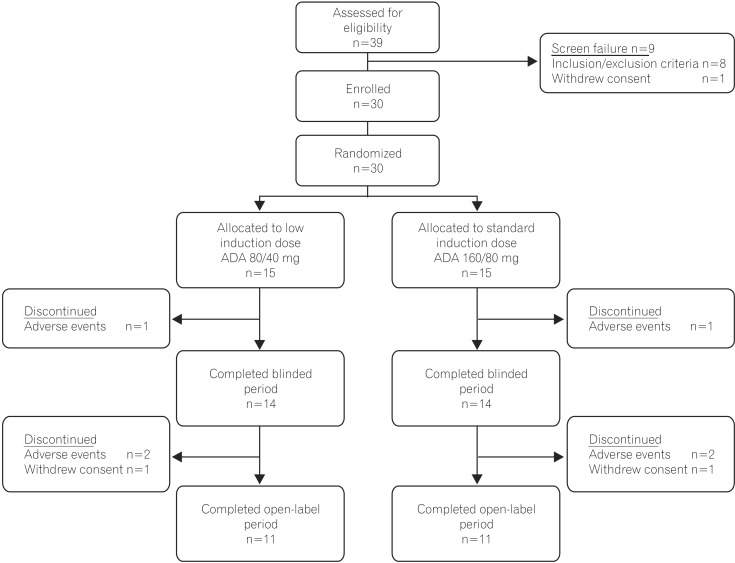
Thirty, adult Chinese patients with CD (CD Activity Index [CDAI] 220-450; high-sensitivity [hs]-C-reactive protein [CRP] ≥3 mg/L) received double-blind adalimumab 160/80 mg or 80/40 mg at weeks 0/2, followed by 40 mg at weeks 4 and 6. An open-label extension period occurred from weeks 8-26; patients received 40 mg adalimumab every other week. Serum adalimumab concentration and change from baseline in fecal calprotectin (FC) were measured during the double-blind period. Clinical remission (CDAI <150), response (decrease in CDAI ≥70 points from baseline), and change from baseline in hs-CRP were assessed through week 26. Nonresponder imputation was used for missing categorical data and last observation carried forward for missing hs-CRP/FC values. No formal hypothesis was tested. Adverse events were monitored.
RESULTS
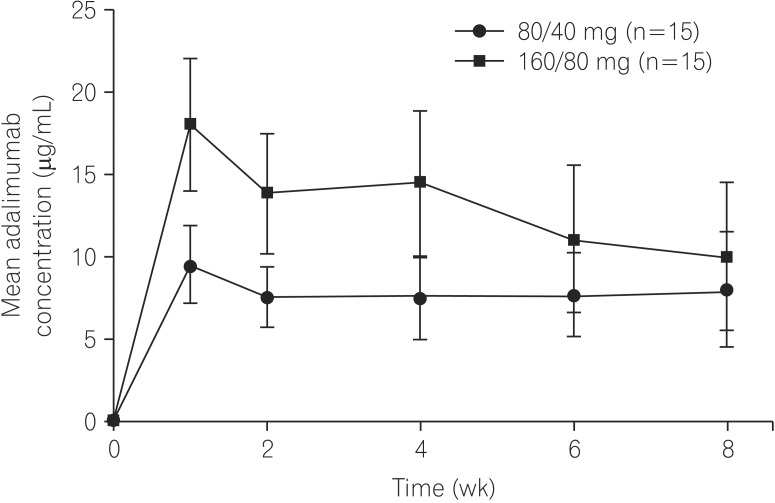
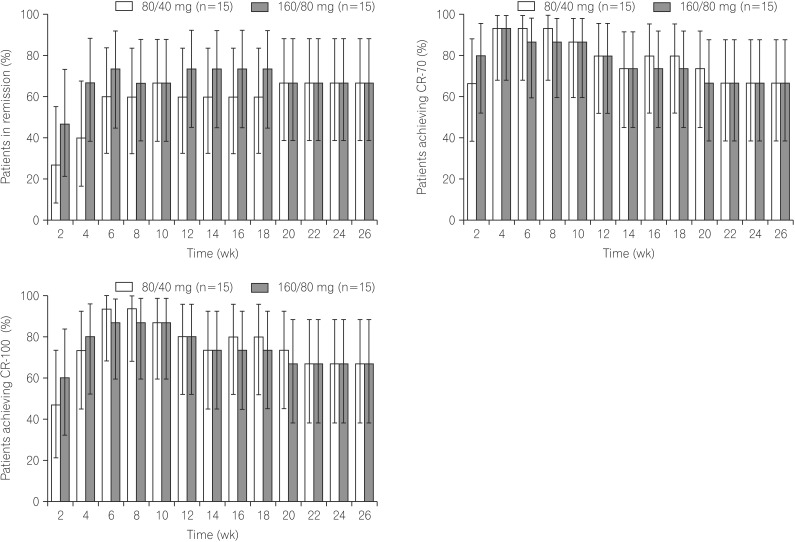
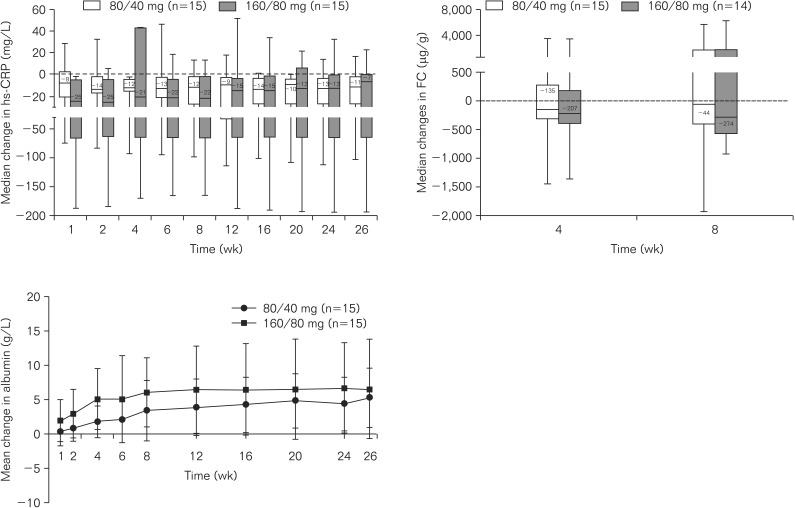
Mean adalimumab serum concentrations during the induction phase were 13.9-18.1 µg/mL (160/80 mg group) and 7.5-9.5 µg/mL (80/40 mg group). During the double-blind period, higher remission/response rates and greater reductions from baseline in hs-CRP and FC were observed with adalimumab 160/80 mg compared to that with 80/40 mg. Adverse event rates were similar among all treatment groups.
CONCLUSIONS
Adalimumab serum concentrations in Chinese patients with CD were comparable to those observed previously in Western and Japanese patients. Clinically meaningful remission rates and improvement in inflammatory markers were achieved with both dosing regimens; changes occurred rapidly with adalimumab 160/80 mg induction therapy. No new safety signals were reported.
MeSH Terms
Figure
Cited by 1 articles
-
Nonimmunity against hepatitis B virus infection in patients newly diagnosed with inflammatory bowel disease
Seong Jae Yeo, Hyun Seok Lee, Byung Ik Jang, Eun Soo Kim, Seong Woo Jeon, Sung Kook Kim, Kyeong Ok Kim, Yoo Jin Lee, Hyun Jik Lee, Kyung Sik Park, Yun Jin Jung, Eun Young Kim, Chang Heon Yang,
Intest Res. 2018;16(3):400-408. doi: 10.5217/ir.2018.16.3.400.
Reference
-
1. Hanauer SB, Sandborn W. Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2001; 96:635–643. PMID: 11280528.
Article2. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013; 145:158–165. PMID: 23583432.
Article3. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013; 28:1148–1153. PMID: 23432198.
Article4. Zhao J, Ng SC, Lei Y, et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of "western" disease. Inflamm Bowel Dis. 2013; 19:1839–1845. PMID: 23669403.5. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280. PMID: 22497584.
Article6. Ooi CJ, Makharia GK, Hilmi I, et al. Asia Pacific Consensus Statements on Crohns disease. Part 1: Definition, diagnosis, and epidemiology: (Asia Pacific Crohn's Disease Consensus-Part 1). J Gastroenterol Hepatol. 2016; 31:45–55. PMID: 25819140.
Article7. Ng SC, Zeng Z, Niewiadomski O, et al. Early course of inflammatory bowel disease in a population-based inception Cohort study from 8 countries in Asia and Australia. Gastroenterology. 2016; 150:86–95. PMID: 26385074.
Article8. Ooi CJ, Makharia GK, Hilmi I, et al. Asia-Pacific consensus statements on Crohn's disease. Part 2: Management. J Gastroenterol Hepatol. 2016; 31:56–68. PMID: 25819311.
Article9. Zheng JJ, Zhi P, Wang YM, et al. Short-term study of infliximab treatment for Crohn's disease in China. J Dig Dis. 2011; 12:105–109. PMID: 21401895.
Article10. Xiao YL, Chen BL, He Y, et al. The clinical and endoscopic efficacy of step-up and top-down infliximab therapy in Crohn's disease. Zhonghua Nei Ke Za Zhi. 2012; 51:100–103. PMID: 22490808.11. Zhou Y, He H, Wang P, et al. Infliximab for the treatment of Crohn's disease: efficacy and safety in a Chinese single-center retrospective study. Eur J Gastroenterol Hepatol. 2015; 27:1270–1275. PMID: 26275085.12. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65. PMID: 17241859.
Article13. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111. PMID: 22326435.
Article14. Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn's disease. J Crohns Colitis. 2012; 6:160–173. PMID: 22325170.
Article15. Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn's disease. Inflamm Bowel Dis. 2013; 19:1112–1122. PMID: 23584130.
Article16. Watanabe M, Hibi T, Mostafa NM, et al. Long-term safety and efficacy of adalimumab in Japanese patients with moderate to severe Crohn's disease. J Crohns Colitis. 2014; 8:1407–1416. PMID: 24874893.
Article17. Huang F, Zhang FC, Bao CD, et al. Adalimumab plus methotrexate for the treatment of rheumatoid arthritis: a multi-center randomized, double-blind, placebo-controlled clinical study. Zhonghua Nei Ke Za Zhi. 2009; 48:916–921. PMID: 20079321.18. Huang F, Gu J, Zhu P, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014; 73:587–594. PMID: 23475983.
Article19. Huang F, Zhang J, Zheng Y, et al. A multicenter, double-blind, randomized, placebo-controlled clinical trial of etanercept treatment of Chinese patients with active ankylosing spondylitis. Zhonghua Nei Ke Za Zhi. 2011; 50:1043–1047. PMID: 22333175.20. Huang F, Zhang J, Huang JL, et al. A multicenter, double-blind, placebo-controlled, randomized, phase III clinical study of etanercept in treatment of ankylosing spondylitis. Zhonghua Nei Ke Za Zhi. 2010; 49:741–745. PMID: 21092442.21. Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004; 10:661–665. PMID: 15472532.
Article22. Zilberman L, Maharshak N, Arbel Y, et al. Correlated expression of high-sensitivity C-reactive protein in relation to disease activity in inflammatory bowel disease: lack of differences between Crohn's disease and ulcerative colitis. Digestion. 2006; 73:205–209. PMID: 16837810.
Article23. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333. PMID: 16472588.
Article24. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007; 56:1232–1239. PMID: 17299059.
Article25. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn's disease. J Crohns Colitis. 2013; 7:213–221. PMID: 22704916.
Article26. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006; 55:426–431. PMID: 16474109.
Article27. Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008; 57:1518–1523. PMID: 18566104.
Article28. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:802–819. PMID: 25964225.
Article29. Colombel JF, Sandborn WJ, Panaccione R, et al. Adalimumab safety in global clinical trials of patients with Crohn's disease. Inflamm Bowel Dis. 2009; 15:1308–1319. PMID: 19434735.
Article30. Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013; 72:517–524. PMID: 22562972.
Article31. Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet. 2014; 383:2057–2064. PMID: 24650955.
Article32. HUMIRA [package insert]. North Chicago, IL: AbbVie Inc.;2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Adalimumab in Korean Patients with Crohn's Disease
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- A Case of Crohn's Disease Showing Favorable Response to Induction and Maintenance Therapy with Methotrexate after Failure of Anti-tumor Necrosis Factor Therapy
- Pulmonary Extraintestinal Manifestation of Crohn's Disease Treated Successfully with Adalimumab
- Eruptive Benign Melanocytic Nevi Formation Following Adalimumab Therapy in a Patient with Crohn's Disease