Lab Med Online.
2018 Oct;8(4):167-170. 10.3343/lmo.2018.8.4.167.
Primary Myelofibrosis with MPL S505N Mutation: The First Case Reported in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Chosun University College of Medicine, Gwangju, Korea. creatgeon@chosun.ac.kr
- KMID: 2421007
- DOI: http://doi.org/10.3343/lmo.2018.8.4.167
Abstract
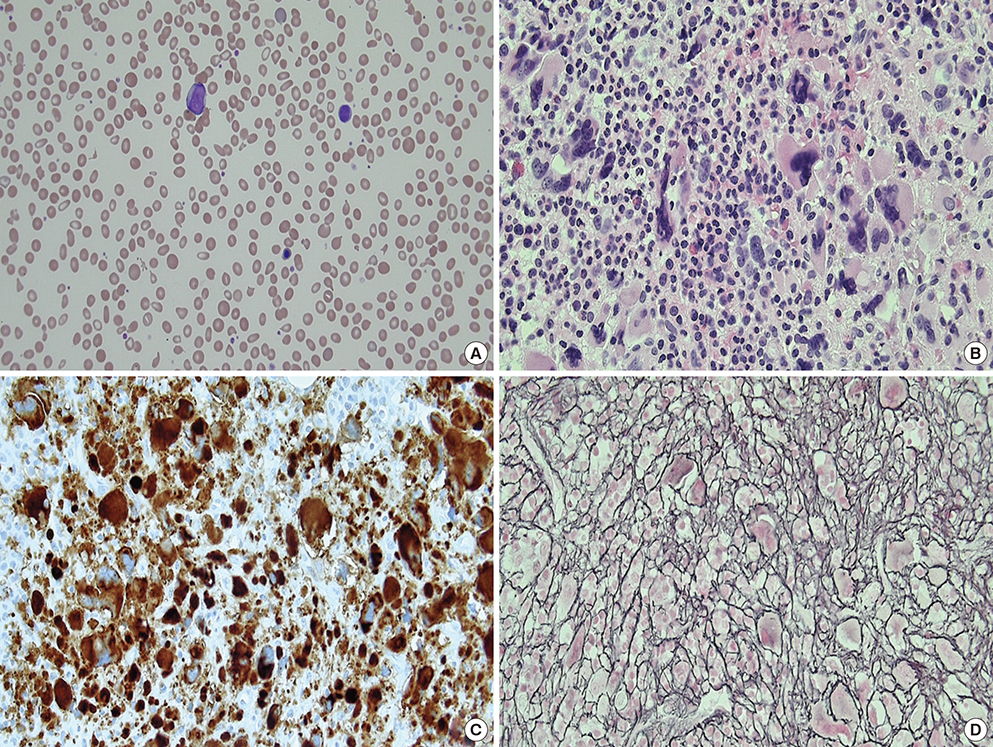
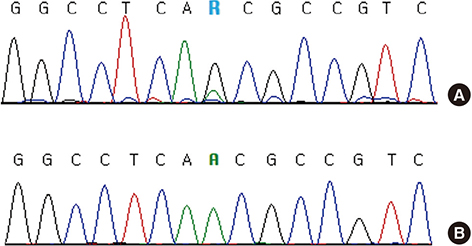
- MPL mutation is an important molecular marker in myeloproliferative neoplasms (MPN). Although MPL W515 is a hot spot for missense mutations in MPN, MPL S505 mutations have been reported in both familial and non-familial MPN. A 72-year-old male visited the hospital, complaining mainly of dizziness and epistaxis. Leukocytosis, anemia, thrombocytopenia, tear drop cells, nucleated RBCs, and myeloblasts were observed in both complete blood cell counts and peripheral blood smears. Bone marrow aspiration failed due to dilution with peripheral blood. BM biopsy indicated hypercellular marrow, megakaryocytic proliferation with atypia, and grade 3 reticulin fibrosis. Conventional cytogenetics results were as follows: 46,XY,del(13)(q12q22)[19]/46,XY[1]. Molecular studies did not detect JAK2 V617F, BCR/ABL translocation, JAK2 exon 12, and CALR exon 9 mutations. The MPL S505N mutation was verified by colony PCR and Sanger sequencing following gene cloning. Based on the above findings, a diagnosis of overt primary myelofibrosis (PMF) was indicated. Mutation studies of buccal and T cells were not conducted. Further, family members were not subjected to mutation studies. Therefore, we were unable to determine whether this mutation was familial or non-familial. Six months after the first visit to the hospital, the patient died due to pneumonia and sepsis. Thrombotic symptoms or major bleeding events did not develop during the survival period following diagnosis of PMF. To the best of our knowledge, this may be the first reported case of PMF with the MPL S505N mutation in Korea.
MeSH Terms
-
Aged
Anemia
Biopsy
Blood Cell Count
Bone Marrow
Clone Cells
Cloning, Organism
Cytogenetics
Diagnosis
Dizziness
Epistaxis
Exons
Fibrosis
Granulocyte Precursor Cells
Hemorrhage
Humans
Korea*
Leukocytosis
Male
Mutation, Missense
Pneumonia
Polymerase Chain Reaction
Primary Myelofibrosis*
Reticulin
Sepsis
T-Lymphocytes
Tears
Thrombocytopenia
Reticulin
Figure
Reference
-
1. Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000; 342:1255–1265.
Article2. Kim SY, Im K, Park SN, Kwon J, Kim JA, Lee DS. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015; 143:635–644.
Article3. Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood. 2017; 129:680–692.
Article4. Ding J, Komatsu H, Wakita A, Kato-Uranishi M, Ito M, Satoh A, et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood. 2004; 103:4198–4200.
Article5. Liu K, Martini M, Rocca B, Amos CI, Teofili L, Giona F, et al. Evidence for a founder effect of the MPL-S505N mutation in eight Italian pedigrees with hereditary thrombocythemia. Haematologica. 2009; 94:1368–1374.
Article6. Teofili L, Giona F, Torti L, Cenci T, Ricerca BM, Rumi C, et al. Hereditary thrombocytosis caused by MPLSer505Asn is associated with a high thrombotic risk, splenomegaly and progression to bone marrow fibrosis. Haematologica. 2010; 95:65–70.
Article7. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365:1054–1061.
Article8. de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994; 369:533–538.
Article9. Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008; 112:141–149.
Article10. Morrell R, Langabeer SE, Smyth L, Perera M, Crotty G. Nonfamilial, MPL S505N-mutated essential thrombocythaemia. Case Rep Hematol. 2013; 2013:729327.11. Lee TS, Kantarjian H, Ma W, Yeh CH, Giles F, Albitar M. Effects of clinically relevant MPL mutations in the transmembrane domain revealed at the atomic level through computational modeling. PLoS One. 2011; 6:e23396.
Article12. Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009; 113:2895–2901.
Article13. Mehrotra M, Patel KP, Chen T, Miranda RN, Wang Y, Zuo Z, et al. Genomic and clinicopathologic features of primary myelofibrosis with isolated 13q deletion. Clin Lymphoma Myeloma Leuk. 2015; 15:496–505.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Frequency and Clinicohematologic Characteristics of MPL W515 Mutations in Patients with Myeloproliferative Neoplasms
- A Rare Case of Essential Thrombocythemia with Coexisting JAK2 and MPL Driver Mutations
- JAK2 V617F and MPL W515L/K Mutations in Korean Patients with Essential Thrombocythemia
- A Case of Primary Autoimmune Myelofibrosis
- JAK2 V617F, MPL, and CALR Mutations in Korean Patients with Essential Thrombocythemia and Primary Myelofibrosis