Transl Clin Pharmacol.
2018 Mar;26(1):25-31. 10.12793/tcp.2018.26.1.25.
Population pharmacokinetic analysis of metformin administered as fixed-dose combination in Korean healthy adults
- Affiliations
-
- 1PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. waystolove@catholic.ac.kr
- 2Department of Clinical Pharmacology and Therapeutics, The Catholic University of Korea Seoul St. Mary's Hospital, Seoul 06591, Korea.
- 3Q-fitter, Inc., Seoul 06591, Korea.
- 4Department of Pharmacology, College of Medicine, the Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2420304
- DOI: http://doi.org/10.12793/tcp.2018.26.1.25
Abstract
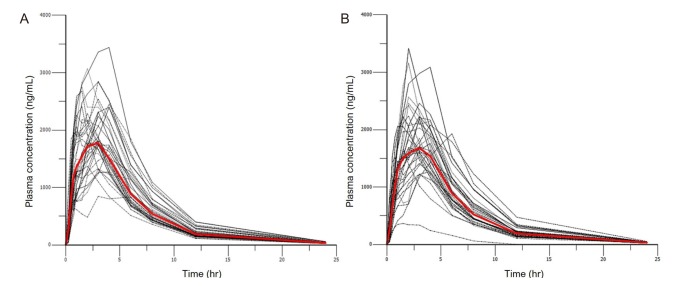
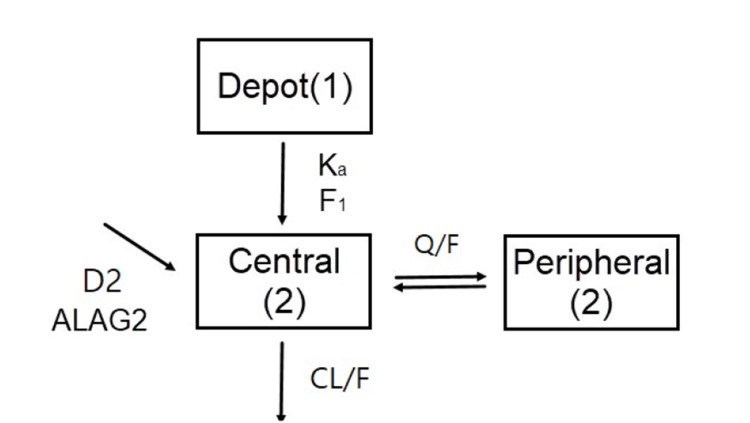
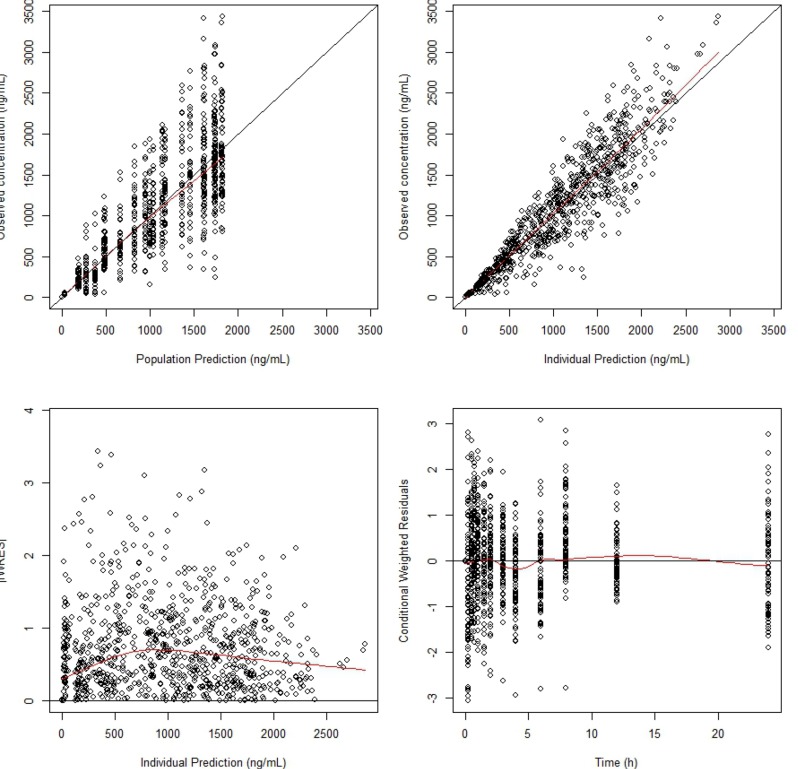
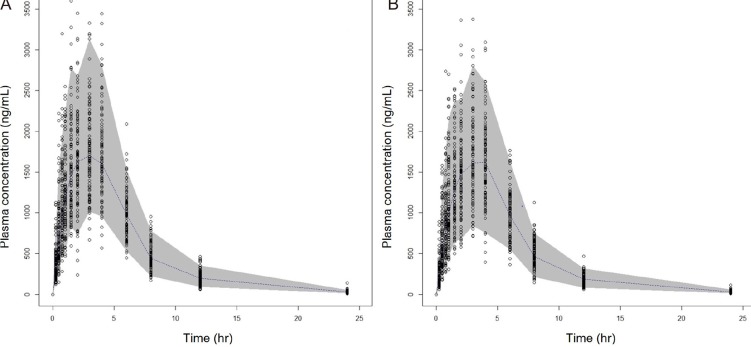
- Metformin, an oral antihyperglycemic agent, is widely used as the first-line pharmacotherapy for type 2 diabetes mellitus (T2DM). It has been in use for several decades as numerous different formulations. However, despite its use, population pharmacokinetic (PK) modeling of metformin is not well developed. The aim of the present study was to evaluate the effect of formulation on PK parameters by developing a population PK model of metformin in Koreans and using this model to assess bioequivalence. We used a comparative PK study of a single agent and a fixed-dose combination of metformin in 36 healthy volunteers. The population PK model of metformin was developed using NONMEM (version 7.3). Visual predictive checks and bootstrap methods were performed to determine the adequacy of the model. The plasma concentration-time profile was best described by a two-compartment, first-order elimination model with first-order absorption followed by zeroorder absorption with lag time. From the covariate analysis, formulation had significant effect (p < 0.01) on relative bioavailability (F = 0.94) and first-order absorption constant (Ka = 0.83), but the difference was within the range of bioequivalence criteria. No other covariate was shown to have significant effect on PK parameters. The PK profile of the disposition phase was consistent with the published literature. However, in the present study, the multiple peaks found during the absorption phase implied the possible diversity of absorption PK profile depending on formulation or population. Unlike traditional bioequivalence analysis, the population PK model reflects formulation differences on specific parameters and reflected simulation can be performed.
Keyword
MeSH Terms
Figure
Reference
-
1. Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011; 50:81–98. DOI: 10.2165/11534750-000000000-00000. PMID: 21241070.
Article2. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996; 30:359–371. PMID: 8743335.
Article3. Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981; 12:235–246. PMID: 7306436.
Article4. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820–827. PMID: 22722338.5. Chitnis SD, Han Y, Yamaguchi M, Mita S, Zhao R, Sunkara G, et al. Population pharmacokinetic modeling and noncompartment analysis demonstrated bioequivalence between metformin component of metformin/vildagliptin fixed-dose combination products and metformin immediate-release tablet source from various countries. Clin Pharmacol Drug Dev. 2016; 5:40–51. PMID: 27119577.6. Davies NM, Takemoto JK, Brocks DR, Yanez JA. Multiple peaking phnomena in pharmacokinetic disposition. Clin Pharmacokinet. 2010; 49:351–377. PMID: 20481648.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Population Pharmacokinetic Analysis of Metformin Administered as Fixed-Dose Combination in Korean Healthy Adults
- Pharmacokinetic Equivalence of the High Dose Strength Fixed-Dose Combination Tablet of Gemigliptin/Metformin Sustained Release (SR) and Individual Component Gemigliptin and Metformin XR Tablets in Healthy Subjects
- Pharmacokinetics and pharmacodynamics of a fixed-dose combination of gemigliptin/metformin sustained release 25/500 mg compared to the loose combination in healthy male subjects
- Use of SGLT2 inhibitor/metformin fixed dose combination in Korea
- Pharmacokinetics of atorvastatin and sustained-release metformin fixed-dose combination tablets: two randomized, open-label, 2-way crossover studies in healthy male subjects under fed conditions