J Korean Neurosurg Soc.
2018 Sep;61(5):559-567. 10.3340/jkns.2017.0297.
Biodegradable Screws Containing Bone Morphogenetic Protein-2 in an Osteoporotic Rat Model
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- 2Laboratory of Stem Cell Therapy, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. neuri71@gmail.com
- 3Department of Biostatistic Consulting, Soon Chun Hyang Medical Center, Bucheon, Korea.
- 4Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Department of Neurosurgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- KMID: 2420063
- DOI: http://doi.org/10.3340/jkns.2017.0297
Abstract
OBJECTIVE
The aim of this study was to evaluate the effect for biodegradable screws containing bone morphogenetic protein-2 (BMP-2) in an osteoporotic rat model.
METHODS
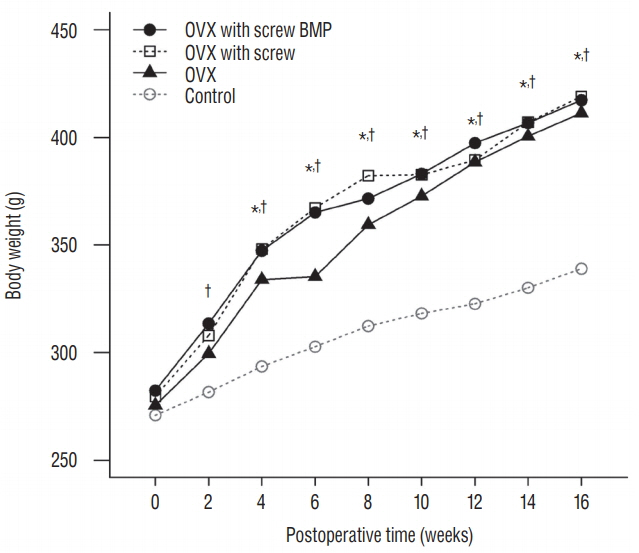
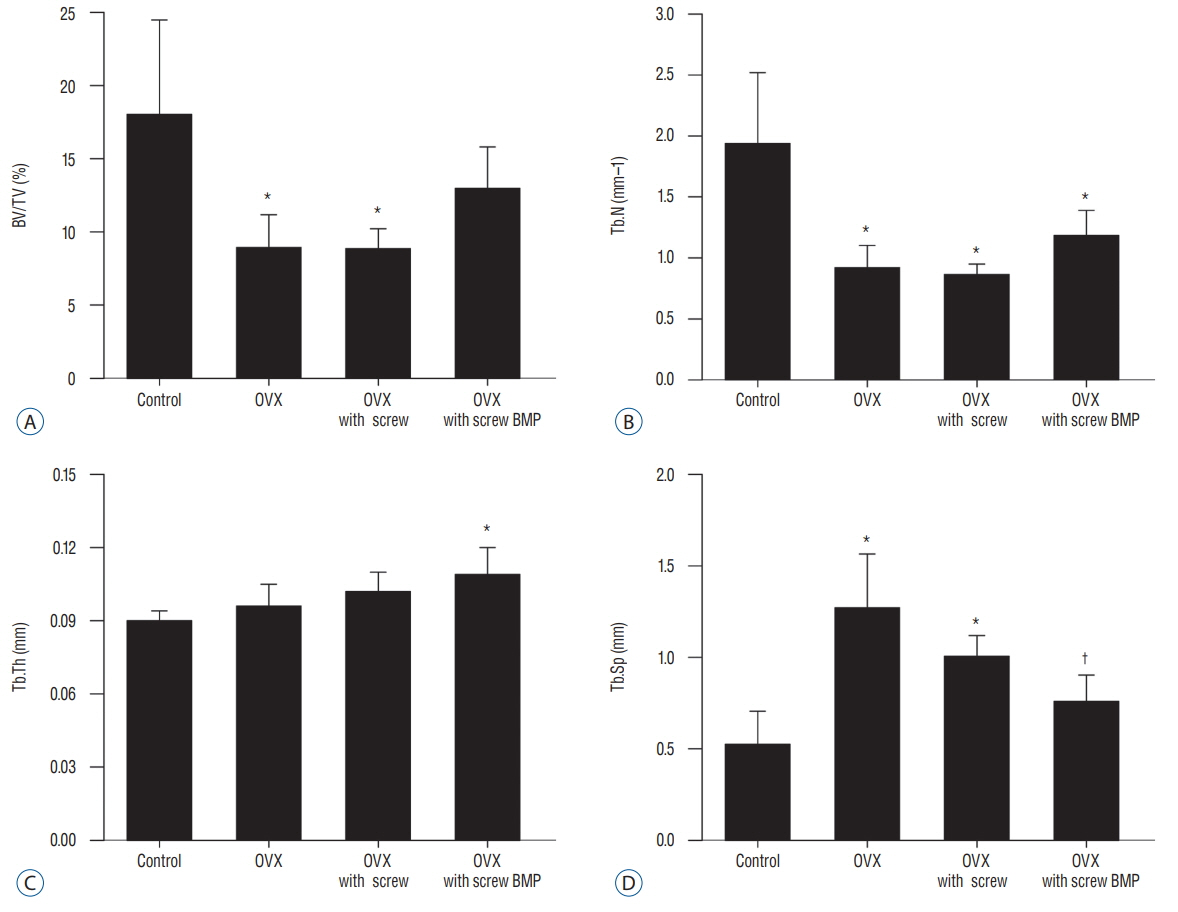
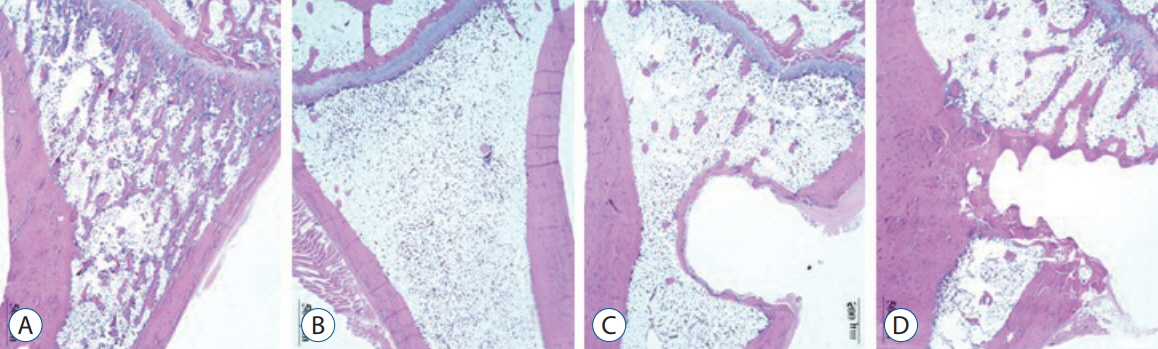
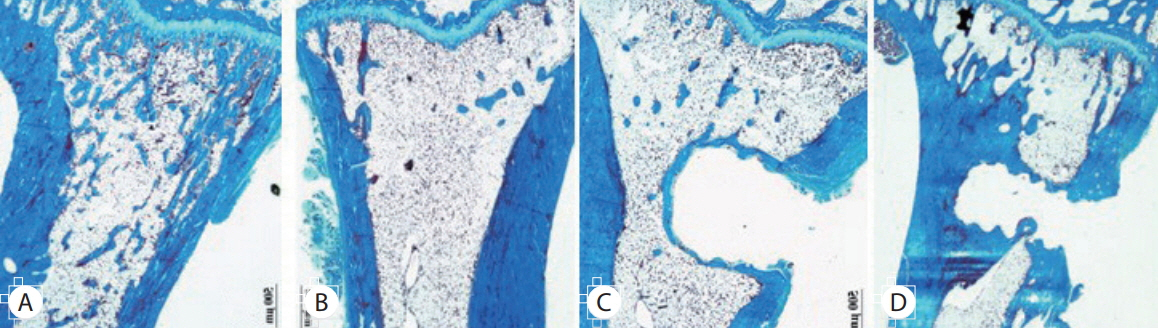
Twenty-four female Wistar rat (250-300 g, 12 weeks of age) were randomized into four groups. Three groups underwent bilateral ovariectomy (OVX). Biodegradable screws with or without BMP-2 were inserted in the proximal tibia in two implantation groups. The extracted proximal metaphysis of the tibiae were scanned by exo-vivo micro-computed tomography. Evaluated parameters included bone mineral density (BMD), trabecular bone volume (BV/TV), trabecular number, trabecular thickness, and trabecular separation (Tb.Sp). The tibia samples were pathologically evaluated by staining with by Hematoxylin and Eosin, and trichrome.
RESULTS
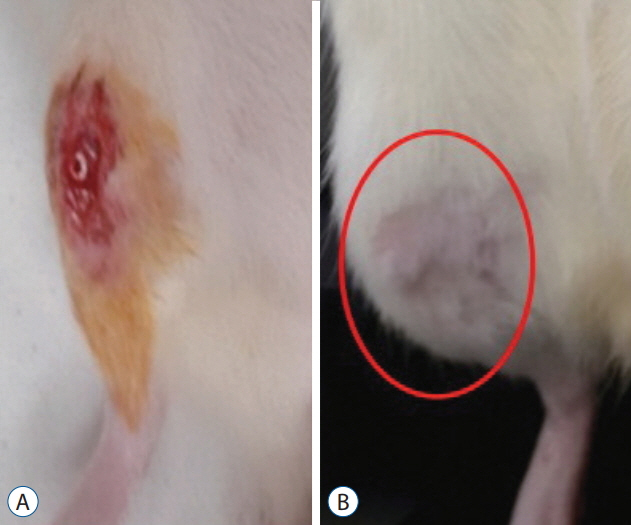
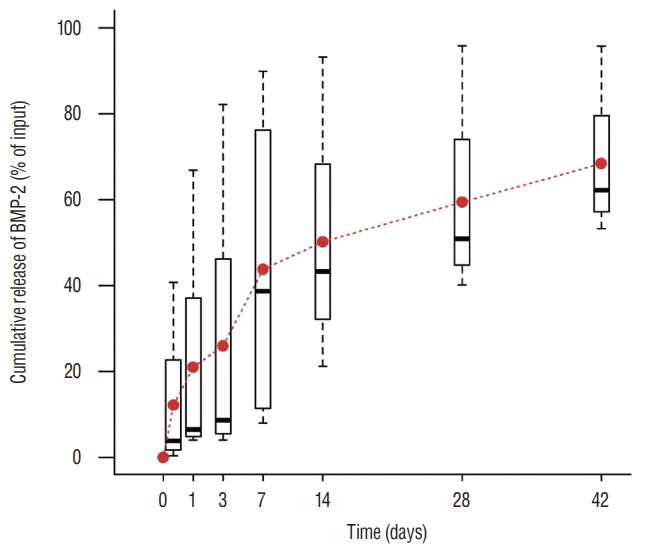
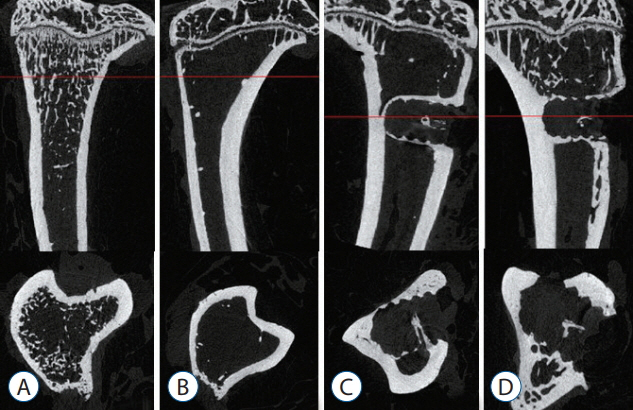
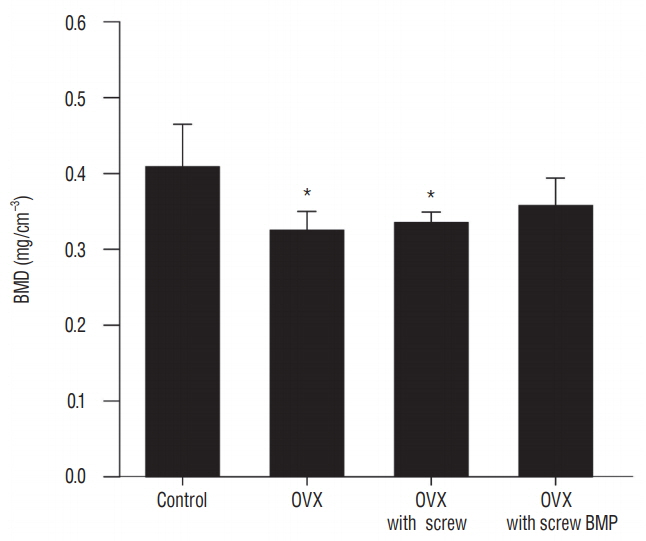
Trabecular formation near screw insertion site was evident only in rats receiving BMP-2 screws. BMD and BV/TV significantly differed between controls and the OVX and OVX with screw groups. However, there were no significant differences between control and OVX with screw BMP groups. Tb.Sp significantly differed between control and OVX and OVX with screw groups (p < 0.05), and between the OVX and OVX with screw BMP group (p < 0.05), with no statistically significant difference between control and OVX with screw BMP groups. Over the 12 weeks after surgery, bone lamellae in direct contact with the screw developed more extensive and thicker trabecular bone around the implant in the OVX with screw BMP group compared to the OVX with screw group.
CONCLUSION
Biodegradable screws containing BMP-2 improve nearby bone conditions and enhance ostoeintegration between the implant and the osteoporotic bone.
MeSH Terms
Figure
Reference
-
References
1. Astrand P, Ahlqvist J, Gunne J, Nilson H. Implant treatment of patients with edentulous jaws: a 20-year follow-up. Clin Implant Dent Relat Res. 10:207–217. 2008.2. Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng Part A. 17:1735–1746. 2011.
Article3. Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 17:513–520. 2002.
Article4. Choi H, Park NJ, Jamiyandorj O, Choi KH, Hong MH, Oh S. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2. J Periodontal Implant Sci. 42:119–126. 2012.
Article5. Cowan CM, Aghaloo T, Chou YF, Walder B, Zhang X, Soo C, et al. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 13:501–512. 2007.
Article6. Duarte PM, César Neto JB, Gonçalves PF, Sallum EA, Nociti jF. Estrogen deficiency affects bone healing around titanium implants: a histometric study in rats. Implant Dent. 12:340–346. 2003.
Article7. Gao Y, Zou S, Liu X, Bao C, Hu J. The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials. 30:1790–1796. 2009.
Article8. Hämmerle CH, Olah AJ, Schmid J, Flückiger L, Gogolewski S, Winkler JR, et al. The biological effect of natural bone mineral on bone neoformation on the rabbit skull. Clin Oral Implants Res. 8:198–207. 1997.
Article9. Herrmann I, Lekholm U, Holm S, Kultje C. Evaluation of patient and implant characteristics as potential prognostic factors for oral implant failures. Int J Oral Maxillofac Implants. 20:220–230. 2005.10. Hyun SJ, Han DK, Choi SH, Chai JK, Cho KS, Kim CK, et al. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J Periodontol. 76:1667–1674. 2005.
Article11. Jakobsen T, Baas J, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local bisphosphonate treatment increases fixation of hydroxyapatite-coated implants inserted with bone compaction. J Orthop Res. 27:189–194. 2009.
Article12. Jung JH, Yun JH, Um YJ, Jung UW, Kim CS, Choi SH, et al. Bone formation of Escherichia coli expressed rhBMP-2 on absorbable collagen block in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 111:298–305. 2011.
Article13. Jung UW, Choi SY, Pang EK, Kim CS, Choi SH, Cho KS. The effect of varying the particle size of beta tricalcium phosphate carrier of recombinant human bone morphogenetic protein-4 on bone formation in rat calvarial defects. J Periodontol. 77:765–772. 2006.
Article14. Lee S, Choi H, Shim JS, Chung MK, Park YB. Comparative study of recombinant human bone morphogenetic protein-2 carriers in rat subcutaneous tissues: Pilot study. Tissue Eng Regen Med. 12:138–146. 2015.
Article15. Li Y, Feng G, Gao Y, Luo E, Liu X, Hu J. Strontium ranelate treatment enhances hydroxyapatite-coated titanium screws fixation in osteoporotic rats. J Orthop Res. 28:578–582. 2010.
Article16. Liang YQ, Qi MC, Xu J, Xu J, Liu HW, Dong W, et al. Low-magnitude high-frequency loading, by whole-body vibration, accelerates early implant osseointegration in ovariectomized rats. Mol Med Rep. 10:2835–2842. 2014.
Article17. Lugero GG, de Falco Caparbo V, Guzzo ML, König B Jr, Jorgetti V. Histomorphometric evaluation of titanium implants in osteoporotic rabbits. Implant Dent. 9:303–309. 2000.
Article18. Mellado-Valero A, Ferrer-García JC, Calvo-Catalá J, Labaig-Rueda C. Implant treatment in patients with osteoporosis. Med Oral Patol Oral Cir Bucal. 15:e52–e57. 2010.
Article19. Qi M, Hu J, Li J, Li J, Dong W, Feng X, et al. Effect of zoledronate acid treatment on osseointegration and fixation of implants in autologous iliac bone grafts in ovariectomized rabbits. Bone. 50:119–127. 2012.
Article20. Qi MC, Zhou XQ, Hu J, Du ZJ, Yang JH, Liu M, et al. Oestrogen replacement therapy promotes bone healing around dental implants in osteoporotic rats. Int J Oral Maxillofac Surg. 33:279–285. 2004.
Article21. Schmoekel H, Schense JC, Weber FE, Grätz KW, Gnägi D, Müller R, et al. Bone healing in the rat and dog with nonglycosylated BMP-2 demonstrating low solubility in fibrin matrices. J Orthop Res. 22:376–381. 2004.
Article22. Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine (Phila Pa 1976). 27:S16–S23. 2002.
Article23. Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 16:329–345. 2005.
Article24. Skoglund B, Holmertz J, Aspenberg P. Systemic and local ibandronate enhance screw fixation. J Orthop Res. 22:1108–1113. 2004.
Article25. Skripitz R, Böhling S, Rüther W, Aspenberg P. Stimulation of implant fixation by parathyroid hormone (1-34)-A histomorphometric comparison of PMMA cement and stainless steel. J Orthop Res. 23:1266–1270. 2005.
Article26. Tägil M, Jeppsson C, Wang JS, Aspenberg P. No augmentation of morselized and impacted bone graft by OP-1 in a weight-bearing model. Acta Orthop Scand. 74:742–748. 2003.
Article27. Urist MR. Bone morphogenetic protein: the molecularization of skeletal system development. J Bone Miner Res. 12:343–346. 1997.
Article28. Wen B, Karl M, Pendrys D, Shafer D, Freilich M, Kuhn L. An evaluation of BMP-2 delivery from scaffolds with miniaturized dental implants in a novel rat mandible model. J Biomed Mater Res B Appl Biomater. 97:315–326. 2011.
Article29. Wikesjö UM, Guglielmoni P, Promsudthi A, Cho KS, Trombelli L, Selvig KA, et al. Periodontal repair in dogs: effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 26:392–400. 1999.
Article30. Yamaguchi K, Masuhara K, Yamasaki S, Nakai T, Fuji T. Cyclic therapy with etidronate has a therapeutic effect against local osteoporosis after cementless total hip arthroplasty. Bone. 33:144–149. 2003.
Article31. Yamazaki Y, Oida S, Akimoto Y, Shioda S. Response of the mouse femoral muscle to an implant of a composite of bone morphogenetic protein and plaster of Paris. Clin Orthop Relat Res. (234):240–249. 1988.
Article32. Zheng LW, Wong MC, Rabie AB, Cheung LK. Evaluation of recombinant human bone morphogenetic protein-2 in mandibular distraction osteogenesis in rabbits: effect of dosage and number of doses on formation of bone. Br J Oral Maxillofac Surg. 44:487–494. 2006.
Article33. Consensus development conference. diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 94:646–650. 1993.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of composite of bone morphogenetic protein and plaster of paris on healing of bone defect in the rat tibia
- Purification of porcine bone morphogenetic protein
- Comparison of the Pullout Strength of Different Pedicle Screw Designs and Augmentation Techniques in an Osteoporotic Bone Model
- The experimental study on the effect of pulsating electromagnetic fields in the osteoinduction induced by bone morphogenetic protein
- Stability of maxillary position after lefort i osteotomy using biodegradable plates and screws